Comparative host protein interactions with HTLV-1 p30 and HTLV-2 p28: insights into difference in pathobiology of human retroviruses
- PMID: 22876852
- PMCID: PMC3464894
- DOI: 10.1186/1742-4690-9-64
Comparative host protein interactions with HTLV-1 p30 and HTLV-2 p28: insights into difference in pathobiology of human retroviruses
Abstract
Background: Human T lymphotropic virus type-1 (HTLV-1) and type 2 (HTLV-2) are closely related human retroviruses, but have unique disease associations. HTLV-1 is the causative agent of an aggressive T-cell leukemia known as adult T-cell leukemia (ATL), HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP), and other inflammatory diseases. HTLV-2 infection has not been clearly associated with any disease condition. Although both viruses can transform T cells in vitro, the HTLV-1 provirus is mainly detected in CD4+ T cells whereas HTLV-2 is mainly detected in CD8+ T cells of infected individuals. HTLV-1 and HTLV-2 encode accessory proteins p30 and p28, respectively, which share partial amino acid homology and are required for viral persistence in vivo. The goal of this study was to identify host proteins interacting with p30 and p28 in order to understand their role in pathogenesis.
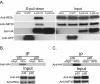
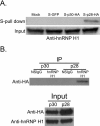
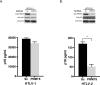
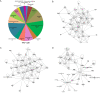
Results: Affinity-tag purification coupled with mass spectrometric (MS) analyses revealed 42 and 22 potential interacting cellular partners of p30 and p28, respectively. Of these, only three cellular proteins, protein arginine methyltransferase 5 (PRMT5), hnRNP K and 60 S ribosomal protein L8 were detected in both p30 and p28 fractions. To validate the proteomic results, four interacting proteins were selected for further analyses using immunoblot assays. In full agreement with the MS analysis two cellular proteins REGγ and NEAF-interacting protein 30 (NIP30) selectively interacted with p30 and not with p28; heterogeneous nuclear ribonucleoprotein H1 (hnRNP H1) bound to p28 and not to p30; and PRMT5 interacted with both p30 and p28. Further studies demonstrated that reduced levels of PRMT5 resulted in decreased HTLV-2 viral gene expression whereas the viral gene expression of HTLV-1 was unchanged.
Conclusion: The comparisons of p30 and p28 host protein interaction proteome showed striking differences with some degree of overlap. PRMT5, one of the host proteins that interacted with both p30 and p28 differentially affected HTLV-1 and HTLV-2 viral gene expression suggesting that PRMT5 is involved at different stages of HTLV-1 and HTLV-2 biology. These findings suggest that distinct host protein interaction profiles of p30 and p28 could, in part, be responsible for differences in HTLV-1 and HTLV-2 pathobiology. This study provides new avenues of investigation into mechanisms of viral infection, tropism and persistence.
Figures

References
-
- Manns A, Blattner WA. The epidemiology of the human T-cell lymphotrophic virus type I and type II: etiologic role in human disease. Transfusion. 1991;31:67–75. - PubMed
-
- Koyanagi Y, Itoyama Y, Nakamura N, Takamatsu K, Kira J, Iwamasa T, Goto I, Yamamoto N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology. 1993;196:25–33. - PubMed
-
- Jones KS, Petrow-Sadowski C, Huang YK, Bertolette DC, Ruscetti FW. Cell-free HTLV-1 infects dendritic cells leading to transmission and transformation of CD4(+) T cells. Nat Med. 2008;14:429–436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

