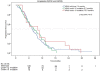
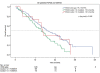
Effect of KRAS codon13 mutations in patients with advanced colorectal cancer (advanced CRC) under oxaliplatin containing chemotherapy. Results from a translational study of the AIO colorectal study group
- PMID: 22876876
- PMCID: PMC3442969
- DOI: 10.1186/1471-2407-12-349
Effect of KRAS codon13 mutations in patients with advanced colorectal cancer (advanced CRC) under oxaliplatin containing chemotherapy. Results from a translational study of the AIO colorectal study group
Abstract
Background: To evaluate the value of KRAS codon 13 mutations in patients with advanced colorectal cancer (advanced CRC) treated with oxaliplatin and fluoropyrimidines.
Methods: Tumor specimens from 201 patients with advanced CRC from a randomized, phase III trial comparing oxaliplatin/5-FU vs. oxaliplatin/capecitabine were retrospectively analyzed for KRAS mutations. Mutation data were correlated to response data (Overall response rate, ORR), progression-free survival (PFS) and overall survival (OS).
Results: 201 patients were analysed for KRAS mutation (61.2% males; mean age 64.2 ± 8.6 years). KRAS mutations were identified in 36.3% of tumors (28.8% in codon 12, 7.4% in codon 13). The ORR in codon 13 patients compared to codon 12 and wild type patients was significantly lower (p = 0.008). There was a tendency for a better overall survival in KRAS wild type patients compared to mutants (p = 0.085). PFS in all patients was not different in the three KRAS genetic groups (p = 0.72). However, we found a marked difference in PFS between patients with codon 12 and 13 mutant tumors treated with infusional 5-FU versus capecitabine based regimens.
Conclusions: Our data suggest that the type of KRAS mutation may be of clinical relevance under oxaliplatin combination chemotherapies without the addition of monoclonal antibodies in particular when overall response rates are important.
Trial registration number: 2002-04-017.
Figures
Similar articles
-
Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104--a randomized trial of the German AIO CRC study group.J Clin Oncol. 2011 Mar 10;29(8):1050-8. doi: 10.1200/JCO.2010.31.1936. Epub 2011 Feb 7. J Clin Oncol. 2011. PMID: 21300933 Clinical Trial.
-
Impact of the specific mutation in KRAS codon 12 mutated tumors on treatment efficacy in patients with metastatic colorectal cancer receiving cetuximab-based first-line therapy: a pooled analysis of three trials.Oncology. 2012;83(5):241-7. doi: 10.1159/000339534. Epub 2012 Aug 29. Oncology. 2012. PMID: 22948721
-
Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study.J Clin Oncol. 2010 Nov 1;28(31):4697-705. doi: 10.1200/JCO.2009.27.4860. Epub 2010 Oct 4. J Clin Oncol. 2010. PMID: 20921465 Clinical Trial.
-
The prevalent KRAS exon 2 c.35 G>A mutation in metastatic colorectal cancer patients: A biomarker of worse prognosis and potential benefit of bevacizumab-containing intensive regimens?Crit Rev Oncol Hematol. 2015 Mar;93(3):190-202. doi: 10.1016/j.critrevonc.2014.10.004. Epub 2014 Oct 16. Crit Rev Oncol Hematol. 2015. PMID: 25459669 Review.
-
Does the Chemotherapy Backbone Impact on the Efficacy of Targeted Agents in Metastatic Colorectal Cancer? A Systematic Review and Meta-Analysis of the Literature.PLoS One. 2015 Aug 14;10(8):e0135599. doi: 10.1371/journal.pone.0135599. eCollection 2015. PLoS One. 2015. PMID: 26275292 Free PMC article.
Cited by
-
Prognostic and predictive roles of KRAS mutation in colorectal cancer.Int J Mol Sci. 2012 Sep 25;13(10):12153-68. doi: 10.3390/ijms131012153. Int J Mol Sci. 2012. PMID: 23202889 Free PMC article. Review.
-
KRAS as a Modulator of the Inflammatory Tumor Microenvironment: Therapeutic Implications.Cells. 2022 Jan 24;11(3):398. doi: 10.3390/cells11030398. Cells. 2022. PMID: 35159208 Free PMC article. Review.
-
Dose KRAS Mutation Status Affect on the Effect of VEGF Therapy in Metastatic Colon Cancer Patients?Cancer Res Treat. 2014 Jan;46(1):48-54. doi: 10.4143/crt.2014.46.1.48. Epub 2014 Jan 15. Cancer Res Treat. 2014. PMID: 24520223 Free PMC article.
-
Mutation status and prognostic value of KRAS and NRAS mutations in Moroccan colon cancer patients: A first report.PLoS One. 2021 Mar 30;16(3):e0248522. doi: 10.1371/journal.pone.0248522. eCollection 2021. PLoS One. 2021. PMID: 33784337 Free PMC article.
-
The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines.Cells. 2020 Jul 25;9(8):1775. doi: 10.3390/cells9081775. Cells. 2020. PMID: 32722384 Free PMC article.
References
-
- Maughan TS, Adams RA, Smith CG. et al.Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377(9783):2103–2114. doi: 10.1016/S0140-6736(11)60613-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous