Specificity and promiscuity in human glutaminase interacting protein recognition: insight from the binding of the internal and C-terminal motif
- PMID: 22876914
- PMCID: PMC3433589
- DOI: 10.1021/bi3008033
Specificity and promiscuity in human glutaminase interacting protein recognition: insight from the binding of the internal and C-terminal motif
Abstract
A large number of cellular processes are mediated by protein-protein interactions, often specified by particular protein binding modules. PDZ domains make up an important class of protein-protein interaction modules that typically bind to the C-terminus of target proteins. These domains act as a scaffold where signaling molecules are linked to a multiprotein complex. Human glutaminase interacting protein (GIP), also known as tax interacting protein 1, is unique among PDZ domain-containing proteins because it is composed almost exclusively of a single PDZ domain rather than one of many domains as part of a larger protein. GIP plays pivotal roles in cellular signaling, protein scaffolding, and cancer pathways via its interaction with the C-terminus of a growing list of partner proteins. We have identified novel internal motifs that are recognized by GIP through combinatorial phage library screening. Leu and Asp residues in the consensus sequence were identified to be critical for binding to GIP through site-directed mutagenesis studies. Structure-based models of GIP bound to two different surrogate peptides determined from nuclear magnetic resonance constraints revealed that the binding pocket is flexible enough to accommodate either the smaller carboxylate (COO(-)) group of a C-terminal recognition motif or the bulkier aspartate side chain (CH(2)COO(-)) of an internal motif. The noncanonical ILGF loop in GIP moves in for the C-terminal motif but moves out for the internal recognition motifs, allowing binding to different partner proteins. One of the peptides colocalizes with GIP within human glioma cells, indicating that GIP might be a potential target for anticancer therapeutics.
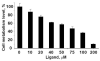
Figures




References
-
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. - PubMed
-
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. - PubMed
-
- Zhong H, Neubig RR. Regulator of G protein signaling proteins: novel multifunctional drug targets. J. Pharmacol. Exp. Ther. 2001;297:837–845. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

