Implantation of undifferentiated and pre-differentiated human neural stem cells in the R6/2 transgenic mouse model of Huntington's disease
- PMID: 22876937
- PMCID: PMC3502570
- DOI: 10.1186/1471-2202-13-97
Implantation of undifferentiated and pre-differentiated human neural stem cells in the R6/2 transgenic mouse model of Huntington's disease
Abstract
Background: Cell therapy is a potential therapeutic approach for several neurodegenetative disease, including Huntington Disease (HD). To evaluate the putative efficacy of cell therapy in HD, most studies have used excitotoxic animal models with only a few studies having been conducted in genetic animal models. Genetically modified animals should provide a more accurate representation of human HD, as they emulate the genetic basis of its etiology.
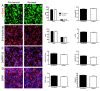
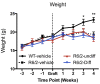
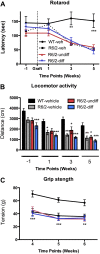
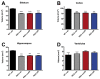
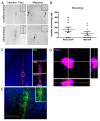
Results: In this study, we aimed to assess the therapeutic potential of a human striatal neural stem cell line (STROC05) implanted in the R6/2 transgenic mouse model of HD. As DARPP-32 GABAergic output neurons are predominately lost in HD, STROC05 cells were also pre-differentiated using purmorphamine, a hedgehog agonist, to yield a greater number of DARPP-32 cells. A bilateral injection of 4.5x105 cells of either undifferentiated or pre-differentiated DARPP-32 cells, however, did not affect outcome compared to a vehicle control injection. Both survival and neuronal differentiation remained poor with a mean of only 161 and 81 cells surviving in the undifferentiated and differentiated conditions respectively. Only a few cells expressed the neuronal marker Fox3.
Conclusions: Although the rapid brain atrophy and short life-span of the R6/2 model constitute adverse conditions to detect potentially delayed treatment effects, significant technical hurdles, such as poor cell survival and differentiation, were also sub-optimal. Further consideration of these aspects is therefore needed in more enduring transgenic HD models to provide a definite assessment of this cell line's therapeutic relevance. However, a combination of treatments is likely needed to affect outcome in transgenic models of HD.
Figures
References
-
- Capetian P, Knoth R, Maciaczyk J, Pantazis G, Ditter M, Bokla L, Landwehrmeyer GB, Volk B, Nikkhah G. Histological findings on fetal striatal grafts in a Huntington's disease patient early after transplantation. Neuroscience. 2009;160(3):661–675. doi: 10.1016/j.neuroscience.2009.02.035. - DOI - PubMed
-
- Gray JA, Grigoryan G, Virley D, Patel S, Sinden JD, Hodges H. Conditionally immortalized, multipotential and multifunctional neural stem cell lines as an approach to clinical transplantation. Cell Transplant. 2000;9(2):153–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

