Low molecular weight amidoximes that act as potent inhibitors of lysine-specific demethylase 1
- PMID: 22876979
- PMCID: PMC3482425
- DOI: 10.1021/jm3002845
Low molecular weight amidoximes that act as potent inhibitors of lysine-specific demethylase 1
Abstract
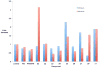
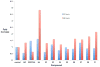
The recently discovered enzyme lysine-specific demethylase 1 (LSD1) plays an important role in the epigenetic control of gene expression, and aberrant gene silencing secondary to LSD1 dysregulation is thought to contribute to the development of cancer. We reported that (bis)guanidines, (bis)biguanides, and their urea- and thiourea isosteres are potent inhibitors of LSD1 and induce the re-expression of aberrantly silenced tumor suppressor genes in tumor cells in vitro. We now report a series of small molecule amidoximes that are moderate inhibitors of recombinant LSD1 but that produce dramatic changes in methylation at the histone 3 lysine 4 (H3K4) chromatin mark, a specific target of LSD1, in Calu-6 lung carcinoma cells. In addition, these analogues increase cellular levels of secreted frizzle-related protein (SFRP) 2, H-cadherin (HCAD), and the transcription factor GATA4. These compounds represent leads for an important new series of drug-like epigenetic modulators with the potential for use as antitumor agents.
Figures






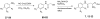
References
-
- Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–483. - PubMed
-
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. - PubMed
-
- Zhang L, Eugeni EE, Parthun MR, Freitas MA. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma. 2003;112(2):77–86. - PubMed
-
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. - PubMed
-
- Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1(4):287–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

