Review of pyronaridine anti-malarial properties and product characteristics
- PMID: 22877082
- PMCID: PMC3483207
- DOI: 10.1186/1475-2875-11-270
Review of pyronaridine anti-malarial properties and product characteristics
Abstract
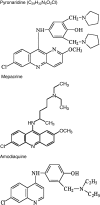
Pyronaridine was synthesized in 1970 at the Institute of Chinese Parasitic Disease and has been used in China for over 30 years for the treatment of malaria. Pyronaridine has high potency against Plasmodium falciparum, including chloroquine-resistant strains. Studies in various animal models have shown pyronaridine to be effective against strains resistant to other anti-malarials, including chloroquine. Resistance to pyronaridine appears to emerge slowly and is further retarded when pyronaridine is used in combination with other anti-malarials, in particular, artesunate. Pyronaridine toxicity is generally less than that of chloroquine, though evidence of embryotoxicity in rodents suggests use with caution in pregnancy. Clinical pharmacokinetic data for pyronaridine indicates an elimination T1/2 of 13.2 and 9.6 days, respectively, in adults and children with acute uncomplicated falciparum and vivax malaria in artemisinin-combination therapy. Clinical data for mono or combined pyronaridine therapy show excellent anti-malarial effects against P. falciparum and studies of combination therapy also show promise against Plasmodium vivax. Pyronaridine has been developed as a fixed dose combination therapy, in a 3:1 ratio, with artesunate for the treatment of acute uncomplicated P. falciparum malaria and blood stage P. vivax malaria with the name of Pyramax® and has received Positive Opinion by European Medicines Agency under the Article 58 procedure.
Figures
References
-
- Zheng XY, Chen C, Gao FH, Zhu PE, Guo HZ. Synthesis of new antimalarial drug pyronaridine and its analogues (author's transl) Yao Xue Xue Bao. 1982;17:118–125. - PubMed
-
- Zheng XY, Xia Y, Gao FH, Chen C. Synthesis of 7351, a new antimalarial drug (author's transl) Yao Xue Xue Bao. 1979;14:736–737. - PubMed
-
- Chen YC, Fleckenstein L. Improved assay method for the determination of pyronaridine in plasma and whole blood by high-performance liquid chromatography for application to clinical pharmacokinetic studies. J Chromatogr B Biomed Sci Appl. 2001;752:39–46. doi: 10.1016/S0378-4347(00)00512-0. - DOI - PubMed
-
- Shao BR. A review of antimalarial drug pyronaridine. Chin Med J (Engl) 1990;103:428–434. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical