Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study
- PMID: 22877847
- PMCID: PMC3856362
- DOI: 10.1016/S1470-2045(12)70285-1
Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study
Abstract
Background: The advent of targeted therapies in the past 7 years has extended median survival for metastatic renal-cell carcinoma. This improvement in clinical outcome has created a need for new, more accurate prognostic measures. We assessed the use of conditional survival--a measure that accounts for elapsed time since treatment initiation--for prognostication in patients with metastatic renal-cell carcinoma treated with first-line VEGF-targeted therapies.
Methods: We obtained data for patients with metastatic renal-cell carcinoma who were treated with a first-line VEGF-targeted therapy between April 7, 2003, and Oct 12, 2010, from our large multi-institutional International mRCC Database Consortium (centres in Canada, the USA, Singapore, Denmark, and South Korea). All histologies, performance statuses, and prognostic risk groups were included. The primary outcome was 2-year conditional survival, defined as the probability of surviving an additional 2 years from a given timepoint since the start of targeted therapy. Secondary analyses included 1-year and 3-year conditional survival, along with stratification of patients by Heng prognostic risk criteria and Karnofsky performance score, and conditional survival based on length of time on therapy. We used the Kaplan-Meier method and a landmark analysis to calculate conditional survival.
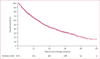
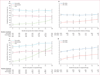
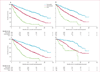
Findings: In the 1673 patients analysed, median follow-up for alive patients was 20·1 months (IQR 9·0-34·4). We recorded an increase in the 2-year conditional survival probability from 44% (95% CI 41-47) at 0 months to 51% (46-55) at 18 months since beginning targeted therapy. When stratified by the Heng prognostic risk criteria defined at therapy initiation, 2-year conditional survival changed little in the favourable and intermediate groups, but in the poor-risk group, 2-year conditional survival improved from 11% (8-15) at 0 months to 33% (18-48) after 18 months. When conditioned on time on targeted therapy from 0 months to 18 months, 2-year conditional survival improved from 44% (41-47) to 68% (60-75) in the overall population and from 74% (68-79) to 90% (77-96) in the favourable group, 49% (45-53) to 57% (45-67) in the intermediate group, and 11% (8-15) to 73% (43-89) in the poor risk group.
Interpretation: Conditional survival is a clinically useful prediction measure that adjusts prognosis of patients with metastatic renal-cell carcinoma on the basis of survival since treatment initiation or therapy duration. Conditional survival might be especially relevant to adjust prognosis for poor-risk patients.
Funding: The Trust Family Fund for Kidney Cancer Research.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
WX, M-HT, and NA declare that they have no conflicts of interest.
Figures
Comment in
-
Difficulty in predicting survival in metastatic renal cancer.Lancet Oncol. 2012 Sep;13(9):859-60. doi: 10.1016/S1470-2045(12)70318-2. Epub 2012 Aug 8. Lancet Oncol. 2012. PMID: 22877849 No abstract available.
-
Commentary on "Conditional survival of patients with metastatic renal cell carcinoma treated with VEGF-targeted therapy: A population-based study." Harshman LC, Xie W, Bjarnason GA, Knox JJ, MacKenzie M, Wood L, Srinivas S, Vaishampayan UN, Tan MH, Rha SY, Donskov F, Agarwal N, Kollmannsberger C, North S, Rini BI, Heng DY, Choueiri TK, Stanford Cancer Institute, Stanford University School of Medicine, Stanford, CA: Lancet Oncol 2012;13(9):927-35 (Epub 2012 Aug 8).Urol Oncol. 2013 Jan;31(1):127-8. doi: 10.1016/j.urolonc.2012.11.006. Urol Oncol. 2013. PMID: 23419724 No abstract available.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Escudier B, Bellmunt J, Negrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28:2144–2150. - PubMed
-
- Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

