Role of TARP interaction in S-SCAM-mediated regulation of AMPA receptors
- PMID: 22878254
- PMCID: PMC3508780
- DOI: 10.4161/chan.21301
Role of TARP interaction in S-SCAM-mediated regulation of AMPA receptors
Abstract
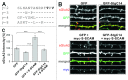
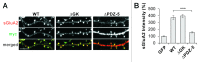
Scaffolding proteins are involved in the incorporation, anchoring, maintenance, and removal of AMPA receptors (AMPARs) at synapses, either through a direct interaction with AMPARs or via indirect association through auxiliary subunits of transmembrane AMPAR regulatory proteins (TARPs). Synaptic scaffolding molecule (S-SCAM) is a newly characterized member of the scaffolding proteins critical for the regulation and maintenance of AMPAR levels at synapses, and directly binds to TARPs through a PDZ interaction. However, the functional significance of S-SCAM-TARP interaction in the regulation of AMPARs has not been tested. Here we show that overexpression of the C-terminal peptide of TARP-γ2 fused to EGFP abolished the S-SCAM-mediated enhancement of surface GluA2 expression. Conversely, the deletion of the PDZ-5 domain of S-SCAM that binds TARPs greatly attenuated the S-SCAM-induced increase of surface GluA2 expression. In contrast, the deletion of the guanylate kinase domain of S-SCAM did not show a significant effect on the regulation of AMPARs. Together, these results suggest that S-SCAM is regulating AMPARs through TARPs.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
