R-loops cause replication impairment and genome instability during meiosis
- PMID: 22878416
- PMCID: PMC3463965
- DOI: 10.1038/embor.2012.119
R-loops cause replication impairment and genome instability during meiosis
Abstract
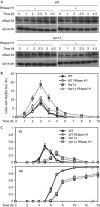
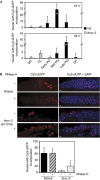
R-loops are harmful structures with a negative impact on transcription and recombination during mitosis, but no information exists for meiosis. We used Saccharomyces cerevisiae and Caenorhabditis elegans THO mutants as a tool to determine the consequences of R-loops in meiosis. We found that both S. cerevisiae and C. elegans THO mutants show defective meiosis and an impairment of premeiotic replication as well as DNA-damage accumulation. Importantly, RNase H partially suppressed the replication impairment and the DNA-damage accumulation. We conclude that R-loops can form during meiosis causing replication impairment with deleterious results.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

