Relating ion channel expression, bifurcation structure, and diverse firing patterns in a model of an identified motor neuron
- PMID: 22878689
- PMCID: PMC6595220
- DOI: 10.1007/s10827-012-0416-6
Relating ion channel expression, bifurcation structure, and diverse firing patterns in a model of an identified motor neuron
Abstract
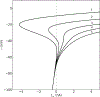
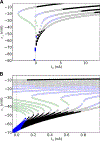
Neurons show diverse firing patterns. Even neurons belonging to a single chemical or morphological class, or the same identified neuron, can display different types of electrical activity. For example, motor neuron MN5, which innervates a flight muscle of adult Drosophila, can show distinct firing patterns under the same recording conditions. We developed a two-dimensional biophysical model and show that a core complement of just two voltage-gated channels is sufficient to generate firing pattern diversity. We propose Shab and DmNa v to be two candidate genes that could encode these core currents, and find that changes in Shab channel expression in the model can reproduce activity resembling the main firing patterns observed in MN5 recordings. We use bifurcation analysis to describe the different transitions between rest and spiking states that result from variations in Shab channel expression, exposing a connection between ion channel expression, bifurcation structure, and firing patterns in models of membrane potential dynamics.
Figures




References
-
- Arhem P, & Blomberg C (2007). Ion channel density and threshold dynamics of repetitive firing in a cortical neuron model. Biosystems, 89(1–3), 117–125. - PubMed
-
- Av-Ron E, Parnas H, Segel L (1991). A minimal biophysical model for an excitable and oscillatory neuron. Biological Cybernetics, 65(6), 487–500. - PubMed
-
- Av-Ron E, Parnas H, Segel LA (1993). A basic biophysical model for bursting neurons. Biological Cybernetics, 69, 87–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

