Optimal management of malignant pleural effusions (results of CALGB 30102)
- PMID: 22878823
- PMCID: PMC5630261
- DOI: 10.6004/jnccn.2012.0102
Optimal management of malignant pleural effusions (results of CALGB 30102)
Abstract
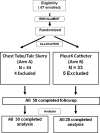
The optimal strategy to achieve palliation of malignant pleural effusions (MPEs) is unknown. This multi-institutional, prospective, randomized trial compares 2 established methods for controlling symptomatic unilateral MPEs. Patients with unilateral MPEs were randomized to either daily tunneled catheter drainage (TCD) or bedside talc pleurodesis (TP). This trial is patterned after a previous randomized trial that showed that bedside TP was equivalent to thoracoscopic TP (CALGB 9334). The primary end point of the current study was combined success: consistent/reliable drainage/pleurodesis, lung expansion, and 30-day survival. A secondary end point, survival with effusion control, was added retrospectively. This trial randomized 57 patients who were similar in terms of age (62 years), active chemotherapy (28%), and histologic diagnosis (lung, 63%; breast, 12%; other/unknown cancers, 25%) to either bedside TP or TCD. Combined success was higher with TCD (62%) than with TP (46%; odds ratio, 5.0; P = .064). Multivariate regression analysis revealed that patients treated with TCD had better 30-day activity without dyspnea scores (8.7 vs. 5.9; P = .036), especially in the subgroup with impaired expansion (9.1 vs. 4.6; P = .042). Patients who underwent TCD had better survival with effusion control at 30 days compared with those who underwent TP (82% vs. 52%, respectively; P = .024). In this prospective randomized trial, TCD achieved superior palliation of unilateral MPEs than TP, particularly in patients with trapped lungs.
References
-
- Marel M, Zrustova M, Stasny B, et al. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest. 1993;104:1486–1489. - PubMed
-
- Putnam JB, Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69:369–375. - PubMed
-
- Putnam JB, Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992–1999. - PubMed
-
- Bresticker MA, Oba J, LoCicero J, III, et al. Optimal pleurodesis: a comparison study. Ann Thorac Surg. 1993;55:364–366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 CA077658/CA/NCI NIH HHS/United States
- CA32291/CA/NCI NIH HHS/United States
- U10 CA045564/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA26806/CA/NCI NIH HHS/United States
- U10 CA059518/CA/NCI NIH HHS/United States
- U10 CA045418/CA/NCI NIH HHS/United States
- CA45564/CA/NCI NIH HHS/United States
- U10 CA047559/CA/NCI NIH HHS/United States
- CA45418/CA/NCI NIH HHS/United States
- U10 CA047642/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- CA35091/CA/NCI NIH HHS/United States
- CA33601/CA/NCI NIH HHS/United States
- U10 CA035091/CA/NCI NIH HHS/United States
- CA77406/CA/NCI NIH HHS/United States
- CA47577/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- CA59518/CA/NCI NIH HHS/United States
- U10 CA021060/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- CA21060/CA/NCI NIH HHS/United States
- CA08025/CA/NCI NIH HHS/United States
- CA74811/CA/NCI NIH HHS/United States
- CA47559/CA/NCI NIH HHS/United States
- CA47642/CA/NCI NIH HHS/United States
- U10 CA074811/CA/NCI NIH HHS/United States
- CA77658/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources