EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors
- PMID: 22879388
- PMCID: PMC3463738
- DOI: 10.1158/1078-0432.CCR-12-1985
EWS-FLI-1-targeted cytotoxic T-cell killing of multiple tumor types belonging to the Ewing sarcoma family of tumors
Abstract
Purpose: The Ewing sarcoma family of tumors (ESFT) comprises a group of aggressive, malignant bone, and soft tissue tumors that predominantly affect children and young adults. These tumors frequently share expression of the EWS-FLI-1 translocation, which is central to tumor survival but not present in healthy cells. In this study, we examined EWS-FLI-1 antigens for their capacity to induce immunity against a range of ESFT types.
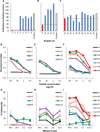
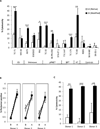
Design: Computer prediction analysis of peptide binding, HLA-A2.1 stabilization assays, and induction of cytotoxic T-lymphocytes (CTL) in immunized HLA-A2.1 transgenic mice were used to assess the immunogenicity of native and modified peptides derived from the fusion region of EWS-FLI-1 type 1. CTL-killing of multiple ESFT family members in vitro, and control of established xenografts in vivo, was assessed. We also examined whether these peptides could induce human CTLs in vitro.
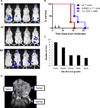
Results: EWS-FLI-1 type 1 peptides were unable to stabilize cell surface HLA-A2.1 and induced weak CTL activity against Ewing sarcoma cells. In contrast, peptides with modified anchor residues induced potent CTL killing of Ewing sarcoma cells presenting endogenous (native) peptides. The adoptive transfer of CTL specific for the modified peptide YLNPSVDSV resulted in enhanced survival of mice with established Ewing sarcoma xenografts. YLNPSVDSV-specific CTL displayed potent killing of multiple ESFT types in vitro: Ewing sarcoma, pPNET, Askin's Tumor, and Biphenotypic sarcoma. Stimulation of human peripheral blood mononuclear cells with YLNPSVDSV peptide resulted in potent CTL-killing.
Conclusions: These data show that YLNPSVDSV peptide is a promising antigen for ESFT immunotherapy and warrants further clinical development.
Conflict of interest statement
The authors disclose no potential conflicts of interest
Figures
References
-
- Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. - PubMed
-
- Lessnick SL, Dei Tos AP, Sorensen PH, Dileo P, Baker LH, Ferrari S, et al. Small round cell sarcomas. Seminars in oncology. 2009;36:338–346. - PubMed
-
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. The New England journal of medicine. 2003;348:694–701. - PubMed
-
- Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing's sarcoma: standard and experimental treatment options. Current treatment options in oncology. 2009;10:126–140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

