Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium
- PMID: 22879416
- PMCID: PMC4113186
- DOI: 10.1167/iovs.12-9917
Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium
Abstract
Purpose: We explored the role of bone morphogenic protein 2 (BMP2) in defocus-induced ocular growth using gene expression changes in RPE as a surrogate.
Methods: Young White-Leghorn chickens were used in this study. Normal gene expression of BMP2 and its receptors was examined in retina, RPE, and choroid, and BMP2 protein expression assessed in the same tissues using Western blots and immunohistochemistry. Quantitative PCR (qPCR) was used to assess the effects of short-term exposure (2 or 48 hours) to monocular +10 and -10 diopter (D) lenses, on RPE gene expression of BMP2 and its receptors. Ocular growth was assessed using A-scan ultrasonography.
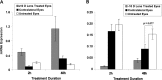
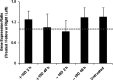
Results: In the eyes of untreated chickens, BMP2 mRNA was expressed more highly in RPE compared to retina and choroid and all three tissues expressed BMP2 protein. The gene expression for all three receptors also was detected in these tissues, with BMPR2 showing highest and BMPR1B lowest expression. BMP2 was up-regulated in the RPE from eyes wearing +10 D lenses, which exhibited shorter than normal vitreous chambers (VCDs) and thickened choroids, while BMP2 was down-regulated in the RPE from eyes wearing -10 D lenses, which developed enlarged VCDs. These treatments did not induce differential expression of BMP receptors in RPE.
Conclusions: That mRNA expression of BMP2 in chick RPE shows bidirectional, defocus sign-dependent changes is suggestive of a role for BMP2 in eye growth regulation, although the diffuse ocular expression of BMP2 and its receptors suggests complex growth-modulatory signal pathways.
Conflict of interest statement
Disclosure:
Figures

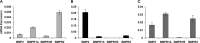




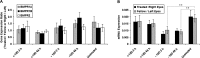
Similar articles
-
Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE.Exp Eye Res. 2013 Apr;109:98-106. doi: 10.1016/j.exer.2013.02.010. Epub 2013 Feb 19. Exp Eye Res. 2013. PMID: 23428741 Free PMC article.
-
Differential gene expression of BMP2 and BMP receptors in chick retina & choroid induced by imposed optical defocus.Vis Neurosci. 2016 Jan;33:E015. doi: 10.1017/S0952523816000122. Vis Neurosci. 2016. PMID: 28359351 Free PMC article.
-
Dynamic BMP gene expression regulation in chick RPE during recovery from short term optical defocus and form-deprivation.PLoS One. 2024 Oct 11;19(10):e0311505. doi: 10.1371/journal.pone.0311505. eCollection 2024. PLoS One. 2024. PMID: 39392817 Free PMC article.
-
Imposed Optical Defocus Induces Isoform-Specific Up-Regulation of TGFβ Gene Expression in Chick Retinal Pigment Epithelium and Choroid but Not Neural Retina.PLoS One. 2016 May 23;11(5):e0155356. doi: 10.1371/journal.pone.0155356. eCollection 2016. PLoS One. 2016. PMID: 27214233 Free PMC article.
-
Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken.Invest Ophthalmol Vis Sci. 2004 Feb;45(2):402-9. doi: 10.1167/iovs.03-0789. Invest Ophthalmol Vis Sci. 2004. PMID: 14744878
Cited by
-
Gene Expression Signatures of Contact Lens-Induced Myopia in Guinea Pig Retinal Pigment Epithelium.Invest Ophthalmol Vis Sci. 2022 Aug 2;63(9):25. doi: 10.1167/iovs.63.9.25. Invest Ophthalmol Vis Sci. 2022. PMID: 36006019 Free PMC article.
-
Insight into the molecular genetics of myopia.Mol Vis. 2017 Dec 31;23:1048-1080. eCollection 2017. Mol Vis. 2017. PMID: 29386878 Free PMC article. Review.
-
Intact globe inflation testing of changes in scleral mechanics in myopia and recovery.Exp Eye Res. 2014 Oct;127:42-8. doi: 10.1016/j.exer.2014.07.004. Epub 2014 Jul 18. Exp Eye Res. 2014. PMID: 25041940 Free PMC article.
-
Postnatal eye size in mice is controlled by SREBP2-mediated transcriptional repression of Lrp2 and Bmp2.Development. 2022 Jul 15;149(14):dev200633. doi: 10.1242/dev.200633. Epub 2022 Jul 14. Development. 2022. PMID: 35833708 Free PMC article.
-
Altered gene expression in tree shrew retina and retinal pigment epithelium produced by short periods of minus-lens wear.Exp Eye Res. 2018 Mar;168:77-88. doi: 10.1016/j.exer.2018.01.005. Epub 2018 Jan 9. Exp Eye Res. 2018. PMID: 29329973 Free PMC article.
References
-
- Kocur I, Resnikoff S. New challenges for VISION 2020. Ophthalmic Epidemiol. 2005;12:291–292 - PubMed
-
- Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–391 - PubMed
-
- Vitale S, Sperduto RD, Ferris FL III. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol. 2009;127:1632–1639 - PubMed
-
- Goldschmidt E. Refraction in the newborn. Acta Ophthalmol (Copenh). 1969;47:570–578 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

