Evidence for bidirectional endocannabinoid transport across cell membranes
- PMID: 22879589
- PMCID: PMC3464571
- DOI: 10.1074/jbc.M112.373241
Evidence for bidirectional endocannabinoid transport across cell membranes
Abstract
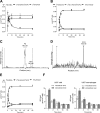
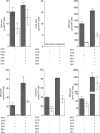
Despite extensive research on the trafficking of anandamide (AEA) across cell membranes, little is known about the membrane transport of other endocannabinoids, such as 2-arachidonoylglycerol (2-AG). Previous studies have provided data both in favor and against a cell membrane carrier-mediated transport of endocannabinoids, using different methodological approaches. Because AEA and 2-AG undergo rapid and almost complete intracellular hydrolysis, we employed a combination of radioligand assays and absolute quantification of cellular and extracellular endocannabinoid levels. In human U937 leukemia cells, 100 nm AEA and 1 μm 2-AG were taken up through a fast and saturable process, reaching a plateau after 5 min. Employing differential pharmacological blockage of endocannabinoid uptake, breakdown, and interaction with intracellular binding proteins, we show that eicosanoid endocannabinoids harboring an arachidonoyl chain compete for a common membrane target that regulates their transport, whereas other N-acylethanolamines did not interfere with AEA and 2-AG uptake. By combining fatty acid amide hydrolase or monoacyl glycerol lipase inhibitors with hydrolase-inactive concentrations of the AEA transport inhibitors UCM707 (1 μm) and OMDM-2 (5 μm), a functional synergism on cellular AEA and 2-AG uptake was observed. Intriguingly, structurally unrelated AEA uptake inhibitors also blocked the cellular release of AEA and 2-AG. We show, for the first time, that UCM707 and OMDM-2 inhibit the bidirectional movement of AEA and 2-AG across cell membranes. Our findings suggest that a putative endocannabinoid cell membrane transporter controls the cellular AEA and 2-AG trafficking and metabolism.
Figures










References
-
- Di Marzo V. (2008) Targeting the endocannabinoid system. To enhance or reduce? Nat. Rev. Drug Discov. 7, 438–455 - PubMed
-
- Cravatt B. F., Giang D. K., Mayfield S. P., Boger D. L., Lerner R. A., Gilula N. B. (1996) Molecular characterization of an enzyme that degrades neuromodulatory fatty acid amides. Nature 384, 83–87 - PubMed
-
- Ueda N., Yamanaka K., Yamamoto S. (2001) Purification and characterization of an acid amidase selective for N-palmitoylethanolamine, a putative endogenous anti-inflammatory substance. J. Biol. Chem. 276, 35552–35557 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

