The metalloprotease meprin β generates amino terminal-truncated amyloid β peptide species
- PMID: 22879596
- PMCID: PMC3460434
- DOI: 10.1074/jbc.M112.395608
The metalloprotease meprin β generates amino terminal-truncated amyloid β peptide species
Abstract
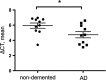
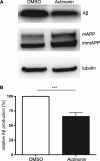
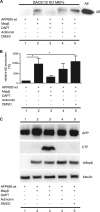
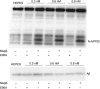
The amyloid β (Aβ) peptide, which is abundantly found in the brains of patients suffering from Alzheimer disease, is central in the pathogenesis of this disease. Therefore, to understand the processing of the amyloid precursor protein (APP) is of critical importance. Recently, we demonstrated that the metalloprotease meprin β cleaves APP and liberates soluble N-terminal APP (N-APP) fragments. In this work, we present evidence that meprin β can also process APP in a manner reminiscent of β-secretase. We identified cleavage sites of meprin β in the amyloid β sequence of the wild type and Swedish mutant of APP at positions p1 and p2, thereby generating Aβ variants starting at the first or second amino acid residue. We observed even higher kinetic values for meprin β than BACE1 for both the wild type and the Swedish mutant APP form. This enzymatic activity of meprin β on APP and Aβ generation was also observed in the absence of BACE1/2 activity using a β-secretase inhibitor and BACE knock-out cells, indicating that meprin β acts independently of β-secretase.
Figures




References
-
- LaFerla F. M., Oddo S. (2005) Alzheimer disease: Aβ, Tau, and synaptic dysfunction. Trends Mol. Med. 11, 170–176 - PubMed
-
- Nagy Z., Esiri M. M., Jobst K. A., Morris J. H., King E. M., McDonald B., Litchfield S., Smith A., Barnetson L., Smith A. D. (1995) Relative roles of plaques and tangles in the dementia of Alzheimer disease: Correlations using three sets of neuropathological criteria. Dementia 6, 21–31 - PubMed
-
- McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer disease. Ann. Neurol. 46, 860–866 - PubMed
-
- Näslund J., Haroutunian V., Mohs R., Davis K. L., Davies P., Greengard P., Buxbaum J. D. (2000) Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. JAMA 283, 1571–1577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

