Unusual stability of messenger RNA in snake venom reveals gene expression dynamics of venom replenishment
- PMID: 22879897
- PMCID: PMC3413681
- DOI: 10.1371/journal.pone.0041888
Unusual stability of messenger RNA in snake venom reveals gene expression dynamics of venom replenishment
Abstract
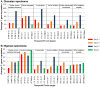
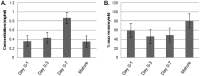
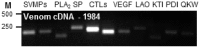
Venom is a critical evolutionary innovation enabling venomous snakes to become successful limbless predators; it is therefore vital that venomous snakes possess a highly efficient venom production and delivery system to maintain their predatory arsenal. Here, we exploit the unusual stability of messenger RNA in venom to conduct, for the first time, quantitative PCR to characterise the dynamics of gene expression of newly synthesised venom proteins following venom depletion. Quantitative PCR directly from venom enables real-time dynamic studies of gene expression in the same animals because it circumvents the conventional requirement to sacrifice snakes to extract mRNA from dissected venom glands. Using qPCR and proteomic analysis, we show that gene expression and protein re-synthesis triggered by venom expulsion peaks between days 3-7 of the cycle of venom replenishment, with different protein families expressed in parallel. We demonstrate that venom re-synthesis occurs very rapidly following depletion of venom stores, presumably to ensure venomous snakes retain their ability to efficiently predate and remain defended from predators. The stability of mRNA in venom is biologically fascinating, and could significantly empower venom research by expanding opportunities to produce transcriptomes from historical venom stocks and rare or endangered venomous species, for new therapeutic, diagnostic and evolutionary studies.
Conflict of interest statement
Figures




Similar articles
-
Venomous snakes of Costa Rica: biological and medical implications of their venom proteomic profiles analyzed through the strategy of snake venomics.J Proteomics. 2014 Jun 13;105:323-39. doi: 10.1016/j.jprot.2014.02.020. Epub 2014 Feb 24. J Proteomics. 2014. PMID: 24576642 Review.
-
Evolution of an arsenal: structural and functional diversification of the venom system in the advanced snakes (Caenophidia).Mol Cell Proteomics. 2008 Feb;7(2):215-46. doi: 10.1074/mcp.M700094-MCP200. Epub 2007 Sep 12. Mol Cell Proteomics. 2008. PMID: 17855442
-
Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly.BMC Genomics. 2015 Aug 28;16:647. doi: 10.1186/s12864-015-1832-6. BMC Genomics. 2015. PMID: 26315097 Free PMC article.
-
Distinct regulatory networks control toxin gene expression in elapid and viperid snakes.BMC Genomics. 2024 Feb 16;25(1):186. doi: 10.1186/s12864-024-10090-y. BMC Genomics. 2024. PMID: 38365592 Free PMC article.
-
Venom gland transcriptomics for identifying, cataloging, and characterizing venom proteins in snakes.Toxicon. 2015 Jan;93:1-10. doi: 10.1016/j.toxicon.2014.10.022. Epub 2014 Oct 29. Toxicon. 2015. PMID: 25448392 Review.
Cited by
-
Third Generation Antivenomics: Pushing the Limits of the In Vitro Preclinical Assessment of Antivenoms.Toxins (Basel). 2017 May 10;9(5):158. doi: 10.3390/toxins9050158. Toxins (Basel). 2017. PMID: 28489039 Free PMC article.
-
Full-Length Venom Protein cDNA Sequences from Venom-Derived mRNA: Exploring Compositional Variation and Adaptive Multigene Evolution.PLoS Negl Trop Dis. 2016 Jun 9;10(6):e0004587. doi: 10.1371/journal.pntd.0004587. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27280639 Free PMC article.
-
Extracellular Vesicles from Bothrops jararaca Venom Are Diverse in Structure and Protein Composition and Interact with Mammalian Cells.Toxins (Basel). 2022 Nov 19;14(11):806. doi: 10.3390/toxins14110806. Toxins (Basel). 2022. PMID: 36422980 Free PMC article.
-
A symmetry or asymmetry: Functional and compositional comparison of venom from the left and right glands of the Indochinese spitting cobra (Naja siamensis).Toxicon X. 2020 Jul 1;7:100050. doi: 10.1016/j.toxcx.2020.100050. eCollection 2020 Sep. Toxicon X. 2020. PMID: 32642644 Free PMC article.
-
Comparison of Four Methods of RNA Extraction and cDNA Synthesis from The Venom of Peruvian Snakes of the Genus Bothrops of Clinical Importance.Int J Mol Sci. 2023 Jul 6;24(13):11161. doi: 10.3390/ijms241311161. Int J Mol Sci. 2023. PMID: 37446341 Free PMC article.
References
-
- Kordis D, Krizaj I, Gubensek F (2002) Functional Diversification of Animal Toxins by Adaptive Evolution. In: Menez A, editor. Perspectives in Molecular Toxinology: John Wiley and Sons Ltd. 401–419.
-
- Mackessy S (1991) Morphology and Ultrastructure of the Venom Glands of the Northern Pacific RattIesnake Crotalus viridis oreganus . Journal of Morphology 208: 109–128. - PubMed
-
- Mackessy S, Baxter LM (2006) Bioweapons synthesis and storage: The venom gland of front-fanged snakes. Zoologischer Anzeiger 245: 147–159.

