Scavenger receptors in human airway epithelial cells: role in response to double-stranded RNA
- PMID: 22879901
- PMCID: PMC3413698
- DOI: 10.1371/journal.pone.0041952
Scavenger receptors in human airway epithelial cells: role in response to double-stranded RNA
Abstract
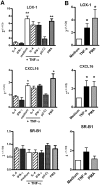
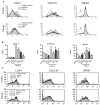
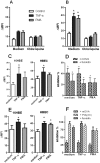
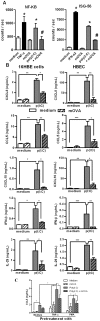
Scavenger receptors and Toll-like receptors (TLRs) cooperate in response to danger signals to adjust the host immune response. The TLR3 agonist double stranded (ds)RNA is an efficient activator of innate signalling in bronchial epithelial cells. In this study, we aimed at defining the role played by scavenger receptors expressed by bronchial epithelial cells in the control of the innate response to dsRNA both in vitro and in vivo. Expression of several scavenger receptor involved in pathogen recognition was first evaluated in human bronchial epithelial cells in steady-state and inflammatory conditions. Their implication in the uptake of dsRNA and the subsequent cell activation was evaluated in vitro by competition with ligand of scavenger receptors including maleylated ovalbumin and by RNA silencing. The capacity of maleylated ovalbumin to modulate lung inflammation induced by dsRNA was also investigated in mice. Exposure to tumor necrosis factor-α increased expression of the scavenger receptors LOX-1 and CXCL16 and the capacity to internalize maleylated ovalbumin, whereas activation by TLR ligands did not. In contrast, the expression of SR-B1 was not modulated in these conditions. Interestingly, supplementation with maleylated ovalbumin limited dsRNA uptake and inhibited subsequent activation of bronchial epithelial cells. RNA silencing of LOX-1 and SR-B1 strongly blocked the dsRNA-induced cytokine production. Finally, administration of maleylated ovalbumin in mice inhibited the dsRNA-induced infiltration and activation of inflammatory cells in bronchoalveolar spaces and lung draining lymph nodes. Together, our data characterize the function of SR-B1 and LOX-1 in bronchial epithelial cells and their implication in dsRNA-induced responses, a finding that might be relevant during respiratory viral infections.
Conflict of interest statement
Figures

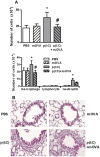

References
-
- Park HS, Suh JH, Kim SS, Kwon OJ (2000) Grain dust induces IL-8 production from bronchial epithelial cells: effect on neutrophil recruitment. Ann Allergy Asthma Immunol 84: 623–627. - PubMed
-
- Pichavant M, Delneste Y, Jeannin P, Fourneau C, Brichet A, et al. (2003) Outer membrane protein A from Klebsiella pneumoniae activates bronchial epithelial cells: implication in neutrophil recruitment. J Immunol 171: 6697–6705. - PubMed
-
- Holgate ST, Lackie P, Wilson S, Roche W, Davies D (2000) Bronchial epithelium as a key regulator of airway allergen sensitization and remodeling in asthma. Am J Respir Crit Care Med 162: S113–117. - PubMed
-
- Takizawa H (1998) Airway epithelial cells as regulators of airway inflammation (Review). Int J Mol Med 1: 367–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

