β-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study
- PMID: 22879929
- PMCID: PMC3412848
- DOI: 10.1371/journal.pone.0042282
β-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: a randomized pilot study
Abstract
Background: Invasive candidiasis (IC) is a devastating disease. While prompt antifungal therapy improves outcomes, empiric treatment based on the presence of fever has little clinical impact. Β-D-Glucan (BDG) is a fungal cell wall component detectable in the serum of patients with early invasive fungal infection (IFI). We evaluated the utility of BDG surveillance as a guide for preemptive antifungal therapy in at-risk intensive care unit (ICU) patients.
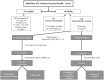
Methods: Patients admitted to the ICU for ≥ 3 days and expected to require at least 2 additional days of intensive care were enrolled. Subjects were randomized in 3:1 fashion to receive twice weekly BDG surveillance with preemptive anidulafungin in response to a positive test or empiric antifungal treatment based on physician preference.
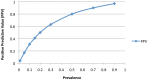
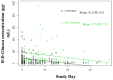
Results: Sixty-four subjects were enrolled, with 1 proven and 5 probable cases of IC identified over a 2.5 year period. BDG levels were higher in subjects with proven/probable IC as compared to those without an IFI (117 pg/ml vs. 28 pg/ml; p<0.001). Optimal assay performance required 2 sequential BDG determinations of ≥ 80 pg/ml to define a positive test (sensitivity 100%, specificity 75%, positive predictive value 30%, negative predictive value 100%). In all, 21 preemptive and 5 empiric subjects received systemic antifungal therapy. Receipt of preemptive antifungal treatment had a significant effect on BDG concentrations (p< 0.001). Preemptive anidulafungin was safe and generally well tolerated with excellent outcome.
Conclusions: BDG monitoring may be useful for identifying ICU patients at highest risk to develop an IFI as well as for monitoring treatment response. Preemptive strategies based on fungal biomarkers warrant further study.
Trial registration: Clinical Trials.gov NCT00672841.
Conflict of interest statement
Figures
References
-
- Falagas ME, Apostolou KE, Pappas VD (2006) Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 25: 419–425. - PubMed
-
- Fridkin SK (2005) Candidemia is costly–plain and simple. Clin Infect Dis 41: 1240–1241. - PubMed
-
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, et al. (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41: 1232–1239. - PubMed
-
- Pfaller MA, Jones RN, Doern GV, Sader HS, Hollis RJ, et al. (1998) International surveillance of bloodstream infections due to Candida species: frequency of occurrence and antifungal susceptibilities of isolates collected in 1997 in the United States, Canada, and South America for the SENTRY Program. The SENTRY Participant Group. J Clin Microbiol 36: 1886–1889. - PMC - PubMed
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39: 309–317. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

