Markov models of use-dependence and reverse use-dependence during the mouse cardiac action potential
- PMID: 22879935
- PMCID: PMC3412869
- DOI: 10.1371/journal.pone.0042295
Markov models of use-dependence and reverse use-dependence during the mouse cardiac action potential
Abstract
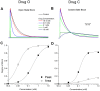
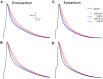
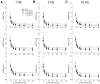
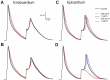
The fast component of the cardiac transient outward current, I(Ktof), is blocked by a number of drugs. The major molecular bases of I(Ktof) are Kv4.2/Kv4.3 voltage-gated potassium channels. Drugs with similar potencies but different blocking mechanisms have differing effects on action potential duration (APD). We used in silico analysis to determine the effect of I(Ktof)-blocking drugs with different blocking mechanisms on mouse ventricular myocytes. We used our existing mouse model of the action potential, and developed 4 new Markov formulations for I(Ktof), I(Ktos), I(Kur), I(Ks). We compared effects of theoretical I(Ktof)-specific channel blockers: (1) a closed state, and (2) an open channel blocker. At concentrations lower or close to IC(50), the drug which bound to the open state always had a much greater effect on APD than the drug which bound to the closed state. At concentrations much higher than IC(50), both mechanisms had similar effects at very low pacing rates. However, an open state binding drug had a greater effect on APD at faster pacing rates, particularly around 10 Hz. In summary, our data indicate that drug effects on APD are strongly dependent not only on IC(50), but also on the drug binding state.
Conflict of interest statement
Figures
 .
.




References
-
- Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, et al. (2003) Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. CardiovascRes 58: 32–45. - PubMed
-
- Roden DM (2004) Drug-induced prolongation of the QT interval. NEnglJMed 350: 1013–1022. - PubMed
-
- Ich (2002) Safety Pharmacology Studies for Assessing the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) By Human Pharmaceuticals. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

