A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus
- PMID: 22879951
- PMCID: PMC3413679
- DOI: 10.1371/journal.pone.0042363
A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus
Abstract
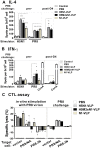
The recent threats of influenza epidemics and pandemics have prioritized the development of a universal vaccine that offers protection against a wider variety of influenza infections. Here, we demonstrate a genetically modified virus-like particle (VLP) vaccine, referred to as H5M2eN1-VLP, that increased the antigenic content of NA and induced rapid recall of antibody against HA(2) after viral infection. As a result, H5M2eN1-VLP vaccination elicited a broad humoral immune response against multiple viral proteins and caused significant protection against homologous RG-14 (H5N1) and heterologous A/California/07/2009 H1N1 (CA/07) and A/PR/8/34 H1N1 (PR8) viral lethal challenges. Moreover, the N1-VLP (lacking HA) induced production of a strong NA antibody that also conferred significant cross protection against H5N1 and heterologous CA/07 but not PR8, suggesting the protection against N1-serotyped viruses can be extended from avian-origin to CA/07 strain isolated in humans, but not to evolutionally distant strains of human-derived. By comparative vaccine study of an HA-based VLP (H5N1-VLP) and NA-based VLPs, we found that H5N1-VLP vaccination induced specific and strong protective antibodies against the HA(1) subunit of H5, thus restricting the breadth of cross-protection. In summary, we present a feasible example of direction of VLP vaccine immunity toward NA and HA(2), which resulted in cross protection against both seasonal and pandemic influenza strains, that could form the basis for future design of a better universal vaccine.
Conflict of interest statement
Figures
Similar articles
-
Neuraminidase-Inhibiting Antibody Titers Correlate with Protection from Heterologous Influenza Virus Strains of the Same Neuraminidase Subtype.J Virol. 2018 Aug 16;92(17):e01006-18. doi: 10.1128/JVI.01006-18. Print 2018 Sep 1. J Virol. 2018. PMID: 29925654 Free PMC article.
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Universal Influenza Virus Neuraminidase Vaccine Elicits Protective Immune Responses against Human Seasonal and Pre-pandemic Strains.J Virol. 2021 Aug 10;95(17):e0075921. doi: 10.1128/JVI.00759-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34160258 Free PMC article.
-
Influenza A viruses: why focusing on M2e-based universal vaccines.Virus Genes. 2011 Feb;42(1):1-8. doi: 10.1007/s11262-010-0547-7. Epub 2010 Nov 17. Virus Genes. 2011. PMID: 21082230 Review.
-
Virus-like particles as universal influenza vaccines.Expert Rev Vaccines. 2012 Aug;11(8):995-1007. doi: 10.1586/erv.12.70. Expert Rev Vaccines. 2012. PMID: 23002980 Free PMC article. Review.
Cited by
-
Use of baculovirus expression system for generation of virus-like particles: successes and challenges.Protein Expr Purif. 2013 Aug;90(2):104-16. doi: 10.1016/j.pep.2013.05.009. Epub 2013 Jun 3. Protein Expr Purif. 2013. PMID: 23742819 Free PMC article. Review.
-
Cross-Reactive Neuraminidase-Inhibiting Antibodies Elicited by Immunization with Recombinant Neuraminidase Proteins of H5N1 and Pandemic H1N1 Influenza A Viruses.J Virol. 2015 Jul;89(14):7224-34. doi: 10.1128/JVI.00585-15. Epub 2015 May 6. J Virol. 2015. PMID: 25948745 Free PMC article.
-
Investigation of Avian Influenza H5N6 Virus-like Particles as a Broad-Spectrum Vaccine Candidate against H5Nx Viruses.Viruses. 2022 Apr 28;14(5):925. doi: 10.3390/v14050925. Viruses. 2022. PMID: 35632667 Free PMC article.
-
Kinetics, Longevity, and Cross-Reactivity of Antineuraminidase Antibody after Natural Infection with Influenza A Viruses.Clin Vaccine Immunol. 2017 Dec 5;24(12):e00248-17. doi: 10.1128/CVI.00248-17. Print 2017 Dec. Clin Vaccine Immunol. 2017. PMID: 29021304 Free PMC article.
-
Cross-Protection Induced by Virus-like Particles Derived from the Influenza B Virus.Biomedicines. 2022 Jul 6;10(7):1618. doi: 10.3390/biomedicines10071618. Biomedicines. 2022. PMID: 35884922 Free PMC article.
References
-
- Skehel JJ, Wiley DC (2000) Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu Rev Biochem 69: 531–569. - PubMed
-
- Cohen FS, Melikyan GB (2001) Implications of a fusion peptide structure. Nat Struct Biol 8: 653–655. - PubMed
-
- Wiley DC, Wilson IA, Skehel JJ (1981) Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289: 373–378. - PubMed
-
- Du L, Zhou Y, Jiang S (2010) Research and development of universal influenza vaccines. Microbes Infect 12: 280–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

