MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection
- PMID: 22879997
- PMCID: PMC3411753
- DOI: 10.1371/journal.pone.0042476
MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection
Abstract
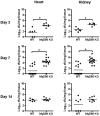
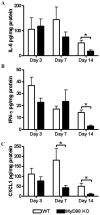
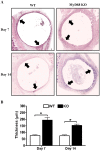
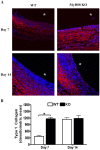
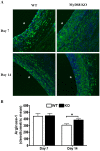
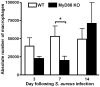
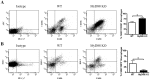
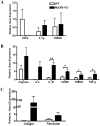
Bacterial biofilms represent a significant therapeutic challenge based on their ability to evade host immune and antibiotic-mediated clearance. Recent studies have implicated IL-1β in biofilm containment, whereas Toll-like receptors (TLRs) had no effect. This is intriguing, since both the IL-1 receptor (IL-1R) and most TLRs impinge on MyD88-dependent signaling pathways, yet the role of this key adaptor in modulating the host response to biofilm growth is unknown. Therefore, we examined the course of S. aureus catheter-associated biofilm infection in MyD88 knockout (KO) mice. MyD88 KO animals displayed significantly increased bacterial burdens on catheters and surrounding tissues during early infection, which coincided with enhanced dissemination to the heart and kidney compared to wild type (WT) mice. The expression of several proinflammatory mediators, including IL-6, IFN-γ, and CXCL1 was significantly reduced in MyD88 KO mice, primarily at the later stages of infection. Interestingly, immunofluorescence staining of biofilm-infected tissues revealed increased fibrosis in MyD88 KO mice concomitant with enhanced recruitment of alternatively activated M2 macrophages. Taken in the context of previous studies with IL-1β, TLR2, and TLR9 KO mice, the current report reveals that MyD88 signaling is a major effector pathway regulating fibrosis and macrophage polarization during biofilm formation. Together these findings represent a novel example of the divergence between TLR and MyD88 action in the context of S. aureus biofilm infection.
Conflict of interest statement
Figures

Similar articles
-
TLR2 and caspase-1 signaling are critical for bacterial containment but not clearance during craniotomy-associated biofilm infection.J Neuroinflammation. 2020 Apr 14;17(1):114. doi: 10.1186/s12974-020-01793-6. J Neuroinflammation. 2020. PMID: 32290861 Free PMC article.
-
Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo.J Immunol. 2011 Jun 1;186(11):6585-96. doi: 10.4049/jimmunol.1002794. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525381 Free PMC article.
-
MyD88-dependent signals are essential for the host immune response in experimental brain abscess.J Immunol. 2007 Apr 1;178(7):4528-37. doi: 10.4049/jimmunol.178.7.4528. J Immunol. 2007. PMID: 17372011 Free PMC article.
-
Immunopathogenesis of Craniotomy Infection and Niche-Specific Immune Responses to Biofilm.Front Immunol. 2021 Feb 23;12:625467. doi: 10.3389/fimmu.2021.625467. eCollection 2021. Front Immunol. 2021. PMID: 33708216 Free PMC article. Review.
-
Deciphering mechanisms of staphylococcal biofilm evasion of host immunity.Front Cell Infect Microbiol. 2012 May 8;2:62. doi: 10.3389/fcimb.2012.00062. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 22919653 Free PMC article. Review.
Cited by
-
The TLR9 Agonist Cobitolimod Induces IL10-Producing Wound Healing Macrophages and Regulatory T Cells in Ulcerative Colitis.J Crohns Colitis. 2020 May 21;14(4):508-524. doi: 10.1093/ecco-jcc/jjz170. J Crohns Colitis. 2020. PMID: 31630153 Free PMC article. Clinical Trial.
-
Staphylococcus epidermidis alters macrophage polarization and phagocytic uptake by extracellular DNA release in vitro.NPJ Biofilms Microbiomes. 2024 Nov 20;10(1):131. doi: 10.1038/s41522-024-00604-7. NPJ Biofilms Microbiomes. 2024. PMID: 39567551 Free PMC article.
-
Phenotypic PIA-Dependent Biofilm Production by Clinical Non-Typeable Staphylococcus aureus Is Not Associated with the Intensity of Inflammation in Mammary Gland: A Pilot Study Using Mouse Mastitis Model.Animals (Basel). 2021 Oct 25;11(11):3047. doi: 10.3390/ani11113047. Animals (Basel). 2021. PMID: 34827779 Free PMC article.
-
Hiding in Plain Sight: Interplay between Staphylococcal Biofilms and Host Immunity.Front Immunol. 2014 Feb 5;5:37. doi: 10.3389/fimmu.2014.00037. eCollection 2014. Front Immunol. 2014. PMID: 24550921 Free PMC article. Review.
-
Forkhead Box O1 Regulates Macrophage Polarization Following Staphylococcus aureus Infection: Experimental Murine Data and Review of the Literature.Clin Rev Allergy Immunol. 2016 Dec;51(3):353-369. doi: 10.1007/s12016-016-8531-1. Clin Rev Allergy Immunol. 2016. PMID: 26924010 Review.
References
-
- Fowler VG, Olsen MK, Corey GR, Woods CW, Cabell CH, et al. (2003) Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 163: 2066–2072. - PubMed
-
- Fitzpatrick F, Humphreys H, O’Gara JP (2005) The genetics of staphylococcal biofilm formation–will a greater understanding of pathogenesis lead to better management of device-related infection? Clin Microbiol Infect 11: 967–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

