Transient knockdown of tyrosine hydroxylase during development has persistent effects on behaviour in adult zebrafish (Danio rerio)
- PMID: 22879998
- PMCID: PMC3411795
- DOI: 10.1371/journal.pone.0042482
Transient knockdown of tyrosine hydroxylase during development has persistent effects on behaviour in adult zebrafish (Danio rerio)
Abstract
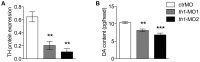
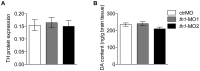
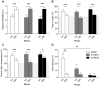
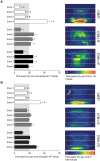
Abnormal dopamine (DA) signaling is often suggested as causative in schizophrenia. The other prominent hypothesis for this disorder, largely driven by epidemiological data, is that certain adverse events during the early stages of brain development increase an individual's risk of developing schizophrenia later in life. However, the clinical and preclinical literature consistently implicates behavioural, cognitive, and pharmacological abnormalities, implying that DA signaling is abnormal in the adult brain. How can we reconcile these two major hypotheses underlying much of the clinical and basic research into schizophrenia? In this study we have transiently knocked down tyrosine hydroxylase (TH, the rate limiting enzyme in DA synthesis) gene expression in the early stages of brain development in zebrafish using morpholinos. We show that by adulthood, TH and DA levels have returned to normal and basic DA-mediated behaviours, such as locomotion, are also normal. However, when they were exposed to a novel environment the levels of freezing and immediate positioning in deeper zones were significantly reduced in these adult fish. The neurochemistry underlying these behaviours is complex, and the exact mechanisms for these abnormal behaviours remains unknown. This study demonstrates that early transient alterations in DA ontogeny can produce persistent alterations in adult brain function and suggests that the zebrafish may be a promising model animal for future studies directed at clarifying the basic neurodevelopmental mechanisms behind complex psychiatric disease.
Conflict of interest statement
Figures

References
-
- Lewis SW, Murray RM (1987) Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res 21: 413–421. - PubMed
-
- Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44: 660–669. - PubMed
-
- Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432. - PubMed
-
- Rapoport JL, Addington AM, Frangou S, Psych MR (2005) The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry 10: 434–449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

