Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells
- PMID: 22879999
- PMCID: PMC3411736
- DOI: 10.1371/journal.pone.0042483
Novel nuclear localization and potential function of insulin-like growth factor-1 receptor/insulin receptor hybrid in corneal epithelial cells
Abstract
Background: Type I insulin-like growth factor receptor (IGF-1R) and insulin receptor (INSR) are highly homologous molecules, which can heterodimerize to form an IGF-1R/INSR hybrid (Hybrid-R). The presence and biological significance of the Hybrid-R in human corneal epithelium has not yet been established. In addition, while nuclear localization of IGF-1R was recently reported in cancer cells and human corneal epithelial cells, the function and profile of nuclear IGF-1R is unknown. In this study, we characterized the nuclear localization and function of the Hybrid-R and the role of IGF-1/IGF-1R and Hybrid-R signaling in the human corneal epithelium.
Methodology/principle findings: IGF-1-mediated signaling and cell growth were examined in a human telomerized corneal epithelial (hTCEpi) cell line using co-immunoprecipitation, immunoblotting and cell proliferation assays. The presence of Hybrid-R in hTCEpi and primary cultured human corneal epithelial cells was confirmed by immunofluorescence and reciprocal immunoprecipitation of whole cell lysates. We found that IGF-1 stimulated Akt and promoted cell growth through IGF-1R activation, which was independent of the Hybrid-R. The presence of Hybrid-R, but not IGF-1R/IGF-1R, was detected in nuclear extracts. Knockdown of INSR by small interfering RNA resulted in depletion of the INSR/INSR and preferential formation of Hybrid-R. Chromatin-immunoprecipitation sequencing assay with anti-IGF-1R or anti-INSR was subsequently performed to identify potential genomic targets responsible for critical homeostatic regulatory pathways.
Conclusion/significance: In contrast to previous reports on nuclear localized IGF-1R, this is the first report identifying the nuclear localization of Hybrid-R in an epithelial cell line. The identification of a nuclear Hybrid-R and novel genomic targets suggests that IGF-1R traffics to the nucleus as an IGF-1R/INSR heterotetrameric complex to regulate corneal epithelial homeostatic pathways. The development of novel therapeutic strategies designed to target the IGF-1/IGF-1R pathway must take into account the modulatory roles IGF-1R/INSR play in the epithelial cell nucleus.
Conflict of interest statement
Figures





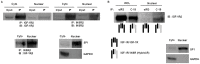
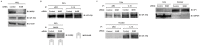
Similar articles
-
Cellular distribution of the IGF-1R in corneal epithelial cells.Exp Eye Res. 2012 Jan;94(1):179-86. doi: 10.1016/j.exer.2011.12.006. Epub 2011 Dec 13. Exp Eye Res. 2012. PMID: 22193032 Free PMC article.
-
Insulin mediates de novo nuclear accumulation of the IGF-1/insulin Hybrid Receptor in corneal epithelial cells.Sci Rep. 2018 Mar 12;8(1):4378. doi: 10.1038/s41598-018-21031-7. Sci Rep. 2018. PMID: 29531349 Free PMC article.
-
Small molecule modulation of insulin receptor-insulin like growth factor-1 receptor heterodimers in human endothelial cells.Mol Cell Endocrinol. 2024 Dec 1;594:112387. doi: 10.1016/j.mce.2024.112387. Epub 2024 Oct 16. Mol Cell Endocrinol. 2024. PMID: 39419341
-
The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease.Front Endocrinol (Lausanne). 2020 Mar 3;11:24. doi: 10.3389/fendo.2020.00024. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32194500 Free PMC article. Review.
-
Insulin receptor (IR) and insulin-like growth factor receptor 1 (IGF-1R) signaling systems: novel treatment strategies for cancer.Med Oncol. 2014 Jan;31(1):805. doi: 10.1007/s12032-013-0805-3. Epub 2013 Dec 14. Med Oncol. 2014. PMID: 24338270 Review.
Cited by
-
Tear Levels of IGFBP-3: A Potential Biomarker for Diabetic Nerve Changes in the Cornea.Eye Contact Lens. 2020 Sep;46(5):319-325. doi: 10.1097/ICL.0000000000000700. Eye Contact Lens. 2020. PMID: 32443005 Free PMC article. Review.
-
Nuclear translocation of IGF-1R via p150(Glued) and an importin-β/RanBP2-dependent pathway in cancer cells.Oncogene. 2015 Apr 23;34(17):2227-38. doi: 10.1038/onc.2014.165. Epub 2014 Jun 9. Oncogene. 2015. PMID: 24909165
-
Nuclear IGF1R Interacts with Regulatory Regions of Chromatin to Promote RNA Polymerase II Recruitment and Gene Expression Associated with Advanced Tumor Stage.Cancer Res. 2018 Jul 1;78(13):3497-3509. doi: 10.1158/0008-5472.CAN-17-3498. Epub 2018 May 7. Cancer Res. 2018. PMID: 29735545 Free PMC article.
-
Insulin receptor preserves mitochondrial function by binding VDAC1 in insulin insensitive mucosal epithelial cells.FASEB J. 2020 Jan;34(1):754-775. doi: 10.1096/fj.201901316RR. Epub 2019 Nov 26. FASEB J. 2020. PMID: 31914671 Free PMC article.
-
Understanding the Key to Targeting the IGF Axis in Cancer: A Biomarker Assessment.Front Oncol. 2015 Jul 8;5:142. doi: 10.3389/fonc.2015.00142. eCollection 2015. Front Oncol. 2015. PMID: 26217584 Free PMC article. Review.
References
-
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM (2008) The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res 14: 6364–6370. - PubMed
-
- De Meyts P, Whittaker J (2002) Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov 1: 769–783. - PubMed
-
- Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, et al. (2002) Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem 277: 39684–39695. - PubMed
-
- Pollak M (2008) Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer 8: 915–928. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

