Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block
- PMID: 22880010
- PMCID: PMC3412845
- DOI: 10.1371/journal.pone.0042512
Waking action of ursodeoxycholic acid (UDCA) involves histamine and GABAA receptor block
Abstract
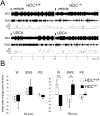
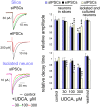
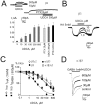
Since ancient times ursodeoxycholic acid (UDCA), a constituent of bile, is used against gallstone formation and cholestasis. A neuroprotective action of UDCA was demonstrated recently in models of Alzheimer's disease and retinal degeneration. The mechanisms of UDCA action in the nervous system are poorly understood. We show now that UDCA promotes wakefulness during the active period of the day, lacking this activity in histamine-deficient mice. In cultured hypothalamic neurons UDCA did not affect firing rate but synchronized the firing, an effect abolished by the GABA(A)R antagonist gabazine. In histaminergic neurons recorded in slices UDCA reduced amplitude and duration of spontaneous and evoked IPSCs. In acutely isolated histaminergic neurons UDCA inhibited GABA-evoked currents and sIPSCs starting at 10 µM (IC(50) = 70 µM) and did not affect NMDA- and AMPA-receptor mediated currents at 100 µM. Recombinant GABA(A) receptors composed of α1, β1-3 and γ2L subunits expressed in HEK293 cells displayed a sensitivity to UDCA similar to that of native GABA(A) receptors. The mutation α1V256S, known to reduce the inhibitory action of pregnenolone sulphate, reduced the potency of UDCA. The mutation α1Q241L, which abolishes GABA(A)R potentiation by several neurosteroids, had no effect on GABA(A)R inhibition by UDCA. In conclusion, UDCA enhances alertness through disinhibition, at least partially of the histaminergic system via GABA(A) receptors.
Conflict of interest statement
Figures




References
-
- Festi D, Montagnani M, Azzaroli F, Lodato F, Mazzella G, et al. (2007) Clinical efficacy and effectiveness of ursodeoxycholic acid in cholestatic liver diseases. Curr Clin Pharmacol 2: 155–177. - PubMed
-
- Woo SJ, Kim JH, Yu HG (2010) Ursodeoxycholic acid and tauroursodeoxycholic acid suppress choroidal neovascularization in a laser-treated rat model. J Ocul Pharmacol Ther 26: 223–229. - PubMed
-
- Boatright JH, Moring AG, McElroy C, Phillips MJ, Do VT, et al. (2006) Tool from ancient pharmacopoeia prevents vision loss. Mol Vis 12: 1706–14.: 1706–1714. - PubMed
-
- Sola S, Amaral JD, Borralho PM, Ramalho RM, Castro RE, et al. (2006) Rodrigues,C.M., Functional modulation of nuclear steroid receptors by tauroursodeoxycholic acid reduces amyloid beta-peptide-induced apoptosis. Mol Endocrinol 20: 2292–2303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

