Presynaptic selectivity of a ligand for serotonin 1A receptors revealed by in vivo PET assays of rat brain
- PMID: 22880045
- PMCID: PMC3413639
- DOI: 10.1371/journal.pone.0042589
Presynaptic selectivity of a ligand for serotonin 1A receptors revealed by in vivo PET assays of rat brain
Abstract
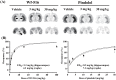
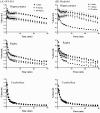
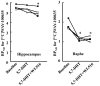
A novel investigational antidepressant with high affinity for the serotonin transporter and the serotonin 1A (5-HT(1A)) receptor, called Wf-516 (structural formula: (2S)-1-[4-(3,4-dichlorophenyl)piperidin-1-yl]-3-[2-(5-methyl-1,3,4-oxadiazol-2-yl)benzo[b]furan-4-yloxy]propan-2-ol monohydrochloride), has been found to exert a rapid therapeutic effect, although the mechanistic basis for this potential advantage remains undetermined. We comparatively investigated the pharmacokinetics and pharmacodynamics of Wf-516 and pindolol by positron emission tomographic (PET) and autoradiographic assays of rat brains in order to elucidate their molecular interactions with presynaptic and postsynaptic 5-HT(1A) receptors. In contrast to the full receptor occupancy by pindolol in PET measurements, the binding of Wf-516 to 5-HT(1A) receptors displayed limited capacity, with relatively high receptor occupancy being achieved in regions predominantly containing presynaptic receptors. This selectivity was further proven by PET scans of neurotoxicant-treated rats deficient in presynaptic 5-HT(1A) receptors. In addition, [(35)S]guanosine 5'-O-[γ-thio]triphosphate autoradiography indicated a partial agonistic ability of Wf-516 for 5-HT(1A) receptors. This finding has lent support to reports that diverse partial agonists for 5-HT(1A) receptors exert high sensitivity for presynaptic components. Thus, the present PET data suggest a relatively high capacity of presynaptic binding sites for partial agonists. Since our in vitro and ex vivo autoradiographies failed to illustrate these distinct features of Wf-516, in vivo PET imaging is considered to be, thus far, the sole method capable of pharmacokinetically demonstrating the unique actions of Wf-516 and similar new-generation antidepressants.
Conflict of interest statement
Figures





Similar articles
-
[18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging.Eur J Nucl Med Mol Imaging. 2010 Mar;37(3):594-605. doi: 10.1007/s00259-009-1274-y. Epub 2009 Sep 30. Eur J Nucl Med Mol Imaging. 2010. PMID: 19789870
-
Utility of small-animal positron emission tomographic imaging of rats for preclinical development of drugs acting on the serotonin transporter.Int J Neuropsychopharmacol. 2009 Sep;12(8):1021-32. doi: 10.1017/S1461145709000042. Epub 2009 Feb 23. Int J Neuropsychopharmacol. 2009. PMID: 19236731
-
5-Hydroxytryptamine(1A) receptor-stimulated [(35)S]GTPgammaS binding in rat brain: absence of regional differences in coupling efficiency.J Pharmacol Exp Ther. 2000 Feb;292(2):684-91. J Pharmacol Exp Ther. 2000. PMID: 10640306
-
Serotonin-1A receptor imaging in recurrent depression: replication and literature review.Nucl Med Biol. 2007 Oct;34(7):865-77. doi: 10.1016/j.nucmedbio.2007.06.008. Nucl Med Biol. 2007. PMID: 17921037 Free PMC article. Review.
-
5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis.Eur J Pharmacol. 2005 Dec 5;526(1-3):125-39. doi: 10.1016/j.ejphar.2005.09.065. Epub 2005 Nov 28. Eur J Pharmacol. 2005. PMID: 16310183 Review.
Cited by
-
Evaluation of serotonin 5-HT(1A) receptors in rodent models using [¹⁸F]mefway PET.Synapse. 2013 Sep;67(9):596-608. doi: 10.1002/syn.21665. Epub 2013 Apr 18. Synapse. 2013. PMID: 23504990 Free PMC article.
-
A small-animal pharmacokinetic/pharmacodynamic PET study of central serotonin 1A receptor occupancy by a potential therapeutic agent for overactive bladder.PLoS One. 2013 Sep 23;8(9):e75040. doi: 10.1371/journal.pone.0075040. eCollection 2013. PLoS One. 2013. PMID: 24086433 Free PMC article.
-
Comparative assessment of (18) F-Mefway as a serotonin 5-HT1A receptor PET imaging agent across species: Rodents, nonhuman primates, and humans.J Comp Neurol. 2016 May 1;524(7):1457-71. doi: 10.1002/cne.23919. Epub 2015 Nov 18. J Comp Neurol. 2016. PMID: 26509362 Free PMC article.
References
-
- Hamed A, Lee A, Ren XS, Miller DR, Cunningham F, et al. (2004) Use of antidepressant medications: are there differences in psychiatric visits among patient treatments in the Veterans Administration? Med Care 42: 551–559. - PubMed
-
- Hansen DG, Søndergaard J, Vach W, Gram LF, Rosholm JU, et al. (2003) Antidepressant drug use in general practice: inter-practice variation and association with practice characteristics. Eur J Clin Pharmacol 59: 143–149. - PubMed
-
- Uchida N, Chong MY, Tan CH, Nagai H, Tanaka M, et al. (2007) International study on antidepressant prescription pattern at 20 teaching hospitals and major psychiatric institutions in East Asia: Analysis of 1898 cases from China, Japan, Korea, Singapore and Taiwan. Psychiatry Clin Neurosci 61: 522–528. - PubMed
-
- Hyttel J (1994) Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clin Psychopharmacol Suppl 1: 19–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

