MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability
- PMID: 22880047
- PMCID: PMC3413640
- DOI: 10.1371/journal.pone.0042596
MMP-13 regulates growth of wound granulation tissue and modulates gene expression signatures involved in inflammation, proteolysis, and cell viability
Abstract
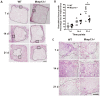
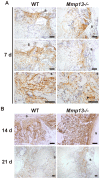
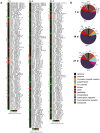
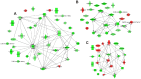
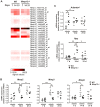
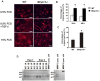
Proteinases play a pivotal role in wound healing by regulating cell-matrix interactions and availability of bioactive molecules. The role of matrix metalloproteinase-13 (MMP-13) in granulation tissue growth was studied in subcutaneously implanted viscose cellulose sponge in MMP-13 knockout (Mmp13(-/-)) and wild type (WT) mice. The tissue samples were harvested at time points day 7, 14 and 21 and subjected to histological analysis and gene expression profiling. Granulation tissue growth was significantly reduced (42%) at day 21 in Mmp13(-/-) mice. Granulation tissue in Mmp13(-/-) mice showed delayed organization of myofibroblasts, increased microvascular density at day 14, and virtual absence of large vessels at day 21. Gene expression profiling identified differentially expressed genes in Mmp13(-/-) mouse granulation tissue involved in biological functions including inflammatory response, angiogenesis, cellular movement, cellular growth and proliferation and proteolysis. Among genes linked to angiogenesis, Adamts4 and Npy were significantly upregulated in early granulation tissue in Mmp13(-/-) mice, and a set of genes involved in leukocyte motility including Il6 were systematically downregulated at day 14. The expression of Pdgfd was downregulated in Mmp13(-/-) granulation tissue in all time points. The expression of matrix metalloproteinases Mmp2, Mmp3, Mmp9 was also significantly downregulated in granulation tissue of Mmp13(-/-) mice compared to WT mice. Mmp13(-/-) mouse skin fibroblasts displayed altered cell morphology and impaired ability to contract collagen gel and decreased production of MMP-2. These results provide evidence for an important role for MMP-13 in wound healing by coordinating cellular activities important in the growth and maturation of granulation tissue, including myofibroblast function, inflammation, angiogenesis, and proteolysis.
Conflict of interest statement
Figures


Similar articles
-
MiR-29a and MiR-140 Protect Chondrocytes against the Anti-Proliferation and Cell Matrix Signaling Changes by IL-1β.Mol Cells. 2016 Feb;39(2):103-10. doi: 10.14348/molcells.2016.2179. Epub 2015 Nov 25. Mol Cells. 2016. PMID: 26608362 Free PMC article.
-
Analysis of fracture healing by large-scale transcriptional profile identified temporal relationships between metalloproteinase and ADAMTS mRNA expression.Matrix Biol. 2006 Jul;25(5):271-81. doi: 10.1016/j.matbio.2006.02.001. Epub 2006 Mar 6. Matrix Biol. 2006. PMID: 16584876
-
Epidermal development and wound healing in matrix metalloproteinase 13-deficient mice.J Invest Dermatol. 2006 Feb;126(2):486-96. doi: 10.1038/sj.jid.5700084. J Invest Dermatol. 2006. PMID: 16374453 Free PMC article.
-
Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice.Br J Dermatol. 2007 Jun;156(6):1163-71. doi: 10.1111/j.1365-2133.2007.07867.x. Epub 2007 Apr 17. Br J Dermatol. 2007. PMID: 17441960
-
Wound healing: insights into autoimmunity, ageing, and cancer ecosystems through inflammation and IL-6 modulation.Front Immunol. 2024 Nov 29;15:1403570. doi: 10.3389/fimmu.2024.1403570. eCollection 2024. Front Immunol. 2024. PMID: 39676864 Free PMC article. Review.
Cited by
-
Multiple myeloma-derived MMP-13 mediates osteoclast fusogenesis and osteolytic disease.J Clin Invest. 2016 May 2;126(5):1759-72. doi: 10.1172/JCI80276. Epub 2016 Apr 4. J Clin Invest. 2016. PMID: 27043283 Free PMC article.
-
Soluble CD83 improves and accelerates wound healing by the induction of pro-resolving macrophages.Front Immunol. 2022 Sep 30;13:1012647. doi: 10.3389/fimmu.2022.1012647. eCollection 2022. Front Immunol. 2022. PMID: 36248909 Free PMC article.
-
Effects of Scoparone on differentiation, adhesion, migration, autophagy and mineralization through the osteogenic signalling pathways.J Cell Mol Med. 2022 Aug;26(16):4520-4529. doi: 10.1111/jcmm.17476. Epub 2022 Jul 7. J Cell Mol Med. 2022. PMID: 35796406 Free PMC article.
-
Varying Properties of Extracellular Matrix Grafts Impact Their Durability and Cell Attachment and Proliferation in an In Vitro Chronic Wound Model.J Tissue Eng Regen Med. 2024 Apr 26;2024:6632276. doi: 10.1155/2024/6632276. eCollection 2024. J Tissue Eng Regen Med. 2024. PMID: 40225755 Free PMC article.
-
Metalloproteinases and Wound Healing.Adv Wound Care (New Rochelle). 2015 Apr 1;4(4):225-234. doi: 10.1089/wound.2014.0581. Adv Wound Care (New Rochelle). 2015. PMID: 25945285 Free PMC article.
References
-
- Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453: 314–321. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

