Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder
- PMID: 22880079
- PMCID: PMC3411809
- DOI: 10.1371/journal.pone.0042676
Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder
Erratum in
- PLoS One. 2013;8(2). doi:10.1371/annotation/85a3fa48-980b-4f95-bb43-b33b1c3e0ac6
Abstract
Background: Meta-analyses have identified serum levels of brain-derived neurotrophic factor (BDNF) as a potential biomarker for major depressive disorder (MDD). However, at the time, commercially available human ELISA kits are unable to distinguish between proBDNF (precursor of BDNF) and mature BDNF because of limited BDNF antibody specificity. In this study, we examined whether serum levels of proBDNF, mature BDNF, and matrix metalloproteinase-9 (MMP-9), which converts proBDNF to mature BDNF, are altered in patients with MDD.
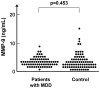
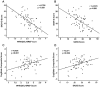
Methodology/principal findings: Sixty-nine patients with MDD and 78 age- and gender-matched healthy subjects were enrolled. Patients were evaluated using 17 items on the Structured Interview Guide for the Hamilton Depression Rating Scale. Cognitive impairment was evaluated using the CogState battery. Serum levels of proBDNF, mature BDNF, and MMP-9 were measured using ELISA kits. Serum levels of mature BDNF in patients with MDD were significantly lower than those of normal controls. In contrast, there was no difference in the serum levels of proBDNF and MMP-9 between patients and normal controls. While neither proBDNF nor mature BDNF serum levels was associated with clinical variables, there were significant correlations between MMP-9 serum levels and the severity of depression, quality of life scores, and social function scores in patients.
Conclusions/significance: These findings suggest that mature BDNF may serve as a biomarker for MDD, and that MMP-9 may play a role in the pathophysiology of MDD. Further studies using larger sample sizes will be needed to investigate these results.
Conflict of interest statement
Figures


Similar articles
-
Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine.Neurosci Lett. 2013 Nov 27;556:37-41. doi: 10.1016/j.neulet.2013.09.059. Epub 2013 Oct 18. Neurosci Lett. 2013. PMID: 24141084
-
Abnormality in serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in mood-stabilized patients with bipolar disorder: a study of two independent cohorts.J Affect Disord. 2014 May;160:1-9. doi: 10.1016/j.jad.2014.01.009. Epub 2014 Feb 24. J Affect Disord. 2014. PMID: 24709015
-
The changes of tPA/PAI-1 system are associated with the ratio of BDNF/proBDNF in major depressive disorder and SSRIs antidepressant treatment.Neuroscience. 2024 Nov 1;559:220-228. doi: 10.1016/j.neuroscience.2024.09.005. Epub 2024 Sep 5. Neuroscience. 2024. PMID: 39244009
-
Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions.Psychiatry Clin Neurosci. 2010 Aug;64(4):341-57. doi: 10.1111/j.1440-1819.2010.02113.x. Psychiatry Clin Neurosci. 2010. PMID: 20653908 Review.
-
BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis.J Affect Disord. 2015 Mar 15;174:432-40. doi: 10.1016/j.jad.2014.11.044. Epub 2014 Nov 29. J Affect Disord. 2015. PMID: 25553404 Review.
Cited by
-
A Bibliometric Analysis of Research on the Role of BDNF in Depression and Treatment.Biomolecules. 2022 Oct 12;12(10):1464. doi: 10.3390/biom12101464. Biomolecules. 2022. PMID: 36291673 Free PMC article. Review.
-
Candidate molecular pathways of white matter vulnerability in the brain of normal aging rhesus monkeys.Geroscience. 2018 Feb;40(1):31-47. doi: 10.1007/s11357-018-0006-2. Epub 2018 Jan 22. Geroscience. 2018. PMID: 29357021 Free PMC article.
-
The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment.Transl Psychiatry. 2017 Apr 4;7(4):e1079. doi: 10.1038/tp.2017.43. Transl Psychiatry. 2017. PMID: 28375203 Free PMC article.
-
Serum brain-derived neurotrophic factor (BDNF) across pregnancy and postpartum: Associations with race, depressive symptoms, and low birth weight.Psychoneuroendocrinology. 2016 Dec;74:69-76. doi: 10.1016/j.psyneuen.2016.08.025. Epub 2016 Aug 27. Psychoneuroendocrinology. 2016. PMID: 27588702 Free PMC article.
-
Genomic and epigenomic insights into nutrition and brain disorders.Nutrients. 2013 Mar 15;5(3):887-914. doi: 10.3390/nu5030887. Nutrients. 2013. PMID: 23503168 Free PMC article.
References
-
- Duman RS, Heninger GR, Nestler EJ (1997) A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597–606. - PubMed
-
- Altar CA (1999) Neurotrophins and depression. Trends Pharmacol Sci 20: 59–61. - PubMed
-
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, et al. (2002) Neurobiology of depression. Neuron 34: 13–25. - PubMed
-
- Martinowich K, Manji H, Lu B (2007) New insights into BDNF function in depression and anxiety. Nat Neurosci 10: 1089–1093. - PubMed
-
- Hashimoto K, Shimizu E, Iyo M (2004) Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev 45: 104–114. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

