Structural context effects in the oxidation of 8-oxo-7,8-dihydro-2'-deoxyguanosine to hydantoin products: electrostatics, base stacking, and base pairing
- PMID: 22880947
- PMCID: PMC3440533
- DOI: 10.1021/ja306077b
Structural context effects in the oxidation of 8-oxo-7,8-dihydro-2'-deoxyguanosine to hydantoin products: electrostatics, base stacking, and base pairing
Abstract
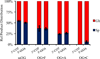
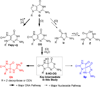
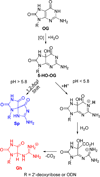
8-Oxo-7,8-dihydroguanine (OG) is the most common base damage found in cells, where it resides in many structural contexts, including the nucleotide pool, single-stranded DNA at transcription forks and replication bubbles, and duplex DNA base-paired with either adenine (A) or cytosine (C). OG is prone to further oxidation to the highly mutagenic hydantoin products spiroiminodihydantoin (Sp) and 5-guanidinohydantoin (Gh) in a sharply pH-dependent fashion within nucleosides. In the present work, studies were conducted to determine how the structural context affects OG oxidation to the hydantoins. These studies revealed a trend in which the Sp yield was greatest in unencumbered contexts, such as nucleosides, while the Gh yield increased in oligodeoxynucleotide (ODN) contexts or at reduced pH. Oxidation of oligomers containing hydrogen-bond modulators (2,6-diaminopurine, N(4)-ethylcytidine) or alteration of the reaction conditions (pH, temperature, and salt) identify base stacking, electrostatics, and base pairing as the drivers of the key intermediate 5-hydroxy-8-oxo-7,8-dihydroguanine (5-HO-OG) partitioning along the two hydantoin pathways, allowing us to propose a mechanism for the observed base-pairing effects. Moreover, these structural effects cause an increase in the effective pK(a) of 5-HO-OG, following an increasing trend from 5.7 in nucleosides to 7.7 in a duplex bearing an OG·C base pair, which supports the context-dependent product yields. The high yield of Gh in ODNs underscores the importance of further study on this lesion. The structural context of OG also determined its relative reactivity toward oxidation, for which the OG·A base pair is ~2.5-fold more reactive than an OG·C base pair, and with the weak one-electron oxidant ferricyanide, the OG nucleoside reactivity is >6000-fold greater than that of OG·C in a duplex, leading to the conclusion that OG in the nucleoside pool should act as a protective agent for OG in the genome.
Figures
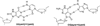







Similar articles
-
Unusual structural features of hydantoin lesions translate into efficient recognition by Escherichia coli Fpg.Biochemistry. 2007 Aug 21;46(33):9355-65. doi: 10.1021/bi602459v. Epub 2007 Jul 27. Biochemistry. 2007. PMID: 17655276 Free PMC article.
-
Removal of hydantoin products of 8-oxoguanine oxidation by the Escherichia coli DNA repair enzyme, FPG.Biochemistry. 2000 Dec 5;39(48):14984-92. doi: 10.1021/bi0017982. Biochemistry. 2000. PMID: 11101315
-
Oxidised guanidinohydantoin (Ghox) and spiroiminodihydantoin (Sp) are major products of iron- and copper-mediated 8-oxo-7,8-dihydroguanine and 8-oxo-7,8-dihydro-2'-deoxyguanosine oxidation.Mol Biosyst. 2005 Dec;1(5-6):373-81. doi: 10.1039/b511756a. Epub 2005 Oct 25. Mol Biosyst. 2005. PMID: 16881006
-
Structure and potential mutagenicity of new hydantoin products from guanosine and 8-oxo-7,8-dihydroguanine oxidation by transition metals.Environ Health Perspect. 2002 Oct;110 Suppl 5(Suppl 5):713-7. doi: 10.1289/ehp.02110s5713. Environ Health Perspect. 2002. PMID: 12426118 Free PMC article. Review.
-
The Influence of 2'-Deoxyguanosine Lesions on the Electronic Properties of OXOG:::C Base Pairs in Ds-DNA: A Comparative Analysis of Theoretical Studies.Molecules. 2024 Aug 8;29(16):3756. doi: 10.3390/molecules29163756. Molecules. 2024. PMID: 39202837 Free PMC article. Review.
Cited by
-
Excision of Oxidatively Generated Guanine Lesions by Competitive DNA Repair Pathways.Int J Mol Sci. 2021 Mar 7;22(5):2698. doi: 10.3390/ijms22052698. Int J Mol Sci. 2021. PMID: 33800059 Free PMC article. Review.
-
Chemistry of ROS-mediated oxidation to the guanine base in DNA and its biological consequences.Int J Radiat Biol. 2022;98(3):452-460. doi: 10.1080/09553002.2021.2003464. Epub 2021 Nov 21. Int J Radiat Biol. 2022. PMID: 34747670 Free PMC article.
-
Pyrimidine base damage is increased in women with BRCA mutations.Cancer Lett. 2013 Sep 28;338(2):267-70. doi: 10.1016/j.canlet.2013.04.001. Epub 2013 Apr 11. Cancer Lett. 2013. PMID: 23583677 Free PMC article.
-
Endonuclease and Exonuclease Activities on Oligodeoxynucleotides Containing Spiroiminodihydantoin Depend on the Sequence Context and the Lesion Stereochemistry.New J Chem. 2013 Nov 1;37(11):3440-3449. doi: 10.1039/C3NJ00418J. New J Chem. 2013. PMID: 24563606 Free PMC article.
-
Nanopore Analysis of the 5-Guanidinohydantoin to Iminoallantoin Isomerization in Duplex DNA.J Org Chem. 2018 Apr 6;83(7):3973-3978. doi: 10.1021/acs.joc.8b00317. Epub 2018 Mar 8. J Org Chem. 2018. PMID: 29490132 Free PMC article.
References
-
- Holliday R. Epigenetics. 2006;1:76–80. - PubMed
-
- Son J, Pang B, McFaline JL, Taghizadeh K, Dedon PC. Mol. Bio. Syst. 2008;4:902–908. - PubMed
-
- Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R, Mecocci P. Ageing Res. Rev. 2009;8:285–305. - PubMed
-
- Aguirre N, Beal MF, Matson WR, Bogdanov MB. Free Rad. Res. 2005;39:383–388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

