ONO-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation
- PMID: 22882023
- PMCID: PMC3594669
- DOI: 10.1111/j.1476-5381.2012.02132.x
ONO-2506 inhibits spike-wave discharges in a genetic animal model without affecting traditional convulsive tests via gliotransmission regulation
Abstract
Background and purpose: Anticonvulsants have been developed according to the traditional neurotransmission imbalance hypothesis. However, the anticonvulsive pharmacotherapy currently available remains unsatisfactory. To develop new antiepileptic drugs with novel antiepileptic mechanisms, we have tested the antiepileptic actions of ONO-2506, a glial modulating agent, and its effects on tripartite synaptic transmission.
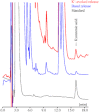
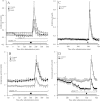
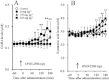
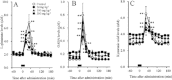
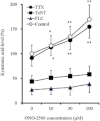
Experimental approach: Dose-dependent effects of ONO-2506 on maximal-electroshock seizure (MES), pentylenetetrazol-induced seizure (PTZ) and epileptic discharge were determined in a genetic model of absence epilepsy in mice (Cacna1a(tm2Nobs/tm2Nobs) strain). Antiepileptic mechanisms of ONO-2506 were analysed by examining the interaction between ONO-2506 and transmission-modulating toxins (tetanus toxin, fluorocitrate, tetrodotoxin) on release of l-glutamate, d-serine, GABA and kynurenic acid in the medial-prefrontal cortex (mPFC) of freely moving rats using microdialysis and primary cultured rat astrocytes.
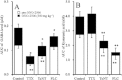
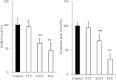
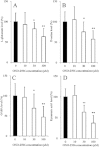
Key results: ONO-2506 inhibited spontaneous epileptic discharges in Cacna1a(tm2Nobs/tm2Nobs) mice without affecting MES or PTZ. Given systemically, ONO-2506 increased basal release of GABA and kynurenic acid in the mPFC through activation of both neuronal and glial exocytosis, but inhibited depolarization-induced releases of all transmitters. ONO-2506 increased basal glial release of kynurenic acid without affecting those of l-glutamate, d-serine or GABA. However, ONO-2506 inhibited AMPA-induced releases of l-glutamate, d-serine, GABA and kynurenic acid.
Conclusions and implications: ONO-2506 did not affect traditional convulsive tests but markedly inhibited epileptic phenomena in the genetic epilepsy mouse model. ONO-2506 enhanced release of inhibitory neuro- and gliotransmitters during the resting stage and inhibited tripartite transmission during the hyperactive stage. The results suggest that ONO-2506 is a novel potential glial-targeting antiepileptic drug.
Linked article: This article is commented on by Onat, pp. 1086-1087 of this issue. To view this commentary visit http://dx.doi.org/10.1111/bph.12050.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

Comment in
-
Astrocytes and absence epilepsy.Br J Pharmacol. 2013 Mar;168(5):1086-7. doi: 10.1111/bph.12050. Br J Pharmacol. 2013. PMID: 23145977 Free PMC article.
References
-
- Asano T, Mori T, Shimoda T, Shinagawa R, Satoh S, Yada N, et al. Arundic acid (ONO-2506) ameliorates delayed ischemic brain damage by preventing astrocytic overproduction of S100B. Curr Drug Targets CNS Neurol Disord. 2005;4:127–142. - PubMed
-
- Blume W. Drug-resistant epilepsy. In: Engel J, Pedley T, editors. Epilepsy: A Comprehensive Text Book. Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1365–1400.
-
- Blumenfeed H, Coulter D. Thalamocortical anatomy and physiology. In: Engel J, Pedley T, editors. Epilepsy: A Comprehensive Text Book. Vol. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 353–366.
-
- Bouwman BM, van Rijn CM. Effects of levetiracetam on spike and wave discharges in WAG/Rij rats. Seizure. 2004;13:591–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

