Osmotic pressure can regulate matrix gene expression in Bacillus subtilis
- PMID: 22882172
- PMCID: PMC3828655
- DOI: 10.1111/j.1365-2958.2012.08201.x
Osmotic pressure can regulate matrix gene expression in Bacillus subtilis
Abstract
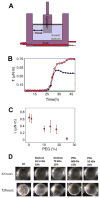
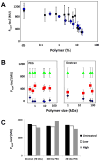
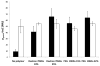
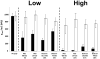
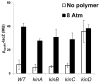
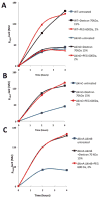
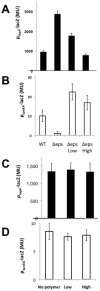
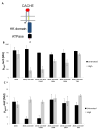
Many bacteria organize themselves into structurally complex communities known as biofilms in which the cells are held together by an extracellular matrix. In general, the amount of extracellular matrix is related to the robustness of the biofilm. Yet, the specific signals that regulate the synthesis of matrix remain poorly understood. Here we show that the matrix itself can be a cue that regulates the expression of the genes involved in matrix synthesis in Bacillus subtilis. The presence of the exopolysaccharide component of the matrix causes an increase in osmotic pressure that leads to an inhibition of matrix gene expression. We further show that non-specific changes in osmotic pressure also inhibit matrix gene expression and do so by activating the histidine kinase KinD. KinD, in turn, directs the phosphorylation of the master regulatory protein Spo0A, which at high levels represses matrix gene expression. Sensing a physical cue such as osmotic pressure, in addition to chemical cues, could be a strategy to non-specifically co-ordinate the behaviour of cells in communities composed of many different species.
© 2012 Blackwell Publishing Ltd.
Figures
Similar articles
-
Phosphorylation of Spo0A by the histidine kinase KinD requires the lipoprotein med in Bacillus subtilis.J Bacteriol. 2011 Aug;193(15):3949-55. doi: 10.1128/JB.05199-11. Epub 2011 May 27. J Bacteriol. 2011. PMID: 21622736 Free PMC article.
-
RemA (YlzA) and RemB (YaaB) regulate extracellular matrix operon expression and biofilm formation in Bacillus subtilis.J Bacteriol. 2009 Jun;191(12):3981-91. doi: 10.1128/JB.00278-09. Epub 2009 Apr 10. J Bacteriol. 2009. PMID: 19363116 Free PMC article.
-
A combination of glycerol and manganese promotes biofilm formation in Bacillus subtilis via histidine kinase KinD signaling.J Bacteriol. 2013 Jun;195(12):2747-54. doi: 10.1128/JB.00028-13. Epub 2013 Apr 5. J Bacteriol. 2013. PMID: 23564171 Free PMC article.
-
Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms.Mol Microbiol. 2014 Aug;93(4):587-98. doi: 10.1111/mmi.12697. Epub 2014 Jul 18. Mol Microbiol. 2014. PMID: 24988880 Free PMC article. Review.
-
Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis.FEMS Microbiol Rev. 2010 Mar;34(2):134-49. doi: 10.1111/j.1574-6976.2009.00199.x. Epub 2009 Nov 23. FEMS Microbiol Rev. 2010. PMID: 20030732 Review.
Cited by
-
The Matrix Reloaded: Probing the Extracellular Matrix Synchronizes Bacterial Communities.J Bacteriol. 2015 Jul;197(13):2092-2103. doi: 10.1128/JB.02516-14. Epub 2015 Mar 30. J Bacteriol. 2015. PMID: 25825428 Free PMC article.
-
The Plant Host Induces Antibiotic Production To Select the Most-Beneficial Colonizers.Appl Environ Microbiol. 2019 Jun 17;85(13):e00512-19. doi: 10.1128/AEM.00512-19. Print 2019 Jul 1. Appl Environ Microbiol. 2019. PMID: 31003984 Free PMC article.
-
Division of Labor during Biofilm Matrix Production.Curr Biol. 2018 Jun 18;28(12):1903-1913.e5. doi: 10.1016/j.cub.2018.04.046. Epub 2018 Jun 7. Curr Biol. 2018. PMID: 29887307 Free PMC article.
-
From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate.PLoS Biol. 2015 Apr 20;13(4):e1002141. doi: 10.1371/journal.pbio.1002141. eCollection 2015 Apr. PLoS Biol. 2015. PMID: 25894589 Free PMC article.
-
The impact of orphan histidine kinases and phosphotransfer proteins on the regulation of clostridial sporulation initiation.mBio. 2024 Apr 10;15(4):e0224823. doi: 10.1128/mbio.02248-23. Epub 2024 Mar 13. mBio. 2024. PMID: 38477571 Free PMC article. Review.
References
-
- Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 1993;7:139–148. - PubMed
-
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

