Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis
- PMID: 22882409
- PMCID: PMC3555482
- DOI: 10.1111/j.1398-9995.2012.02875.x.
Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis
Abstract
Background: Human induced pluripotent stem cells (iPSCs) possess remarkable self-renewal capacity and the potential to differentiate into novel cell types, such as mesenchymal stem cells (MSCs). iPSC-MSCs have been shown to enhance tissue regeneration and attenuate tissue ischaemia; however, their contribution to the immune regulation of Th2-skewed allergic rhinitis (AR) and asthma remains unclear.
Objective: This study compared the immunomodulatory effects of iPSC-MSCs and bone marrow-derived MSCs (BM-MSCs) on lymphocyte proliferation, T-cell phenotypes and cytokine production in peripheral blood mononuclear cells (PBMCs) in patients with AR, and investigated the possible molecular mechanisms underlying the immunomodulatory properties of iPSC-MSCs.
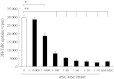
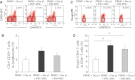
Methods: In co-cultures of PBMCs with iPSC-MSCs or BM-MSCs, lymphocyte proliferation was evaluated using 3H-thymidine (3H-TdR) uptake, carboxyfluorescein diacetate, succinimidyl ester (CFDA-SE) assays; the regulatory T-cell (Treg) phenotype was determined by flow cytometry, and cytokine levels were measured using an enzyme-linked immunosorbent assay. The immunomodulatory properties of both MSCs were further evaluated using NS398 and transwell experiments.
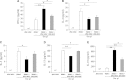
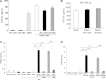
Results: Similar to BM-MSCs, we determined that iPSC-MSCs significantly inhibit lymphocyte proliferation and promote Treg response in PBMCs (P < 0.05). Accordingly, the cytokine milieu (IFN-γ, IL-4, IL-5, IL-10 and IL-13) in the supernatants of PBMCs changed significantly (P < 0.05). The immunomodulatory properties of iPSC-MSCs and BM-MSCs were associated with prostaglandin E2 (PGE2) production and cell-cell contact.
Conclusions: These data demonstrate that iPSC-MSCs are capable of modulating T-cell phenotypes towards Th2 suppression through inducing Treg expansion, suggesting that iPSC-MSCs can be used as an alternative candidate to adult MSCs to treat allergic airway diseases.
© 2012 John Wiley & Sons A/S.
Figures


References
-
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl. 86):8–160. - PubMed
-
- Shi HZ, Qin XJ. CD4CD25 regulatory T lymphocytes in allergy and asthma. Allergy. 2005;60:986–995. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

