O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability
- PMID: 22883232
- PMCID: PMC3480732
- DOI: 10.1016/j.cmet.2012.07.006
O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability
Abstract
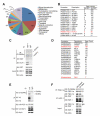
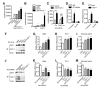
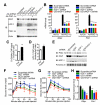
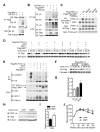
A major cause of hyperglycemia in diabetic patients is inappropriate hepatic gluconeogenesis. PGC-1α is a master regulator of gluconeogenesis, and its activity is controlled by various posttranslational modifications. A small portion of glucose metabolizes through the hexosamine biosynthetic pathway, which leads to O-linked β-N-acetylglucosamine (O-GlcNAc) modification of cytoplasmic and nuclear proteins. Using a proteomic approach, we identified a broad variety of proteins associated with O-GlcNAc transferase (OGT), among which host cell factor C1 (HCF-1) is highly abundant. HCF-1 recruits OGT to O-GlcNAcylate PGC-1α, and O-GlcNAcylation facilitates the binding of the deubiquitinase BAP1, thus protecting PGC-1α from degradation and promoting gluconeogenesis. Glucose availability modulates gluconeogenesis through the regulation of PGC-1α O-GlcNAcylation and stability by the OGT/HCF-1 complex. Hepatic knockdown of OGT and HCF-1 improves glucose homeostasis in diabetic mice. These findings define the OGT/HCF-1 complex as a glucose sensor and key regulator of gluconeogenesis, shedding light on new strategies for treating diabetes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Capotosti F, Guernier S, Lammers F, Waridel P, Cai Y, Jin J, Conaway JW, Conaway RC, Herr W. O-GlcNAc Transferase Catalyzes Site-Specific Proteolysis of HCF-1. Cell. 2011;144:376–388. - PubMed
-
- Chikanishi T, Fujiki R, Hashiba W, Sekine H, Yokoyama A, Kato S. Glucose-induced expression of MIP-1 genes requires O-GlcNAc transferase in monocytes. Biochem Biophys Res Commun. 2010;394:865–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK089098/DK/NIDDK NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
- P30 AG021342/AG/NIA NIH HHS/United States
- P01 DK057751/DK/NIDDK NIH HHS/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
- P30-DK34989/DK/NIDDK NIH HHS/United States
- P30-DK045735/DK/NIDDK NIH HHS/United States
- P30 DK034989/DK/NIDDK NIH HHS/United States
- P01-DK057751/DK/NIDDK NIH HHS/United States
- P41 GM103533/GM/NIGMS NIH HHS/United States
- P30-AG021342/AG/NIA NIH HHS/United States
- R01-DK089098/DK/NIDDK NIH HHS/United States
- P41-RR011823/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

