Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome
- PMID: 22883234
- PMCID: PMC3418541
- DOI: 10.1016/j.cmet.2012.07.005
Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome
Abstract
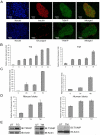
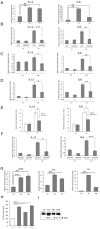
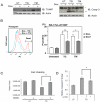
Recent clinical and experimental evidence suggests that endoplasmic reticulum (ER) stress contributes to the life-and-death decisions of β cells during the progression of type 1 and type 2 diabetes. Although crosstalk between inflammation and ER stress has been suggested to play a significant role in β cell dysfunction and death, a key molecule connecting ER stress to inflammation has not been identified. Here we report that thioredoxin-interacting protein (TXNIP) is a critical signaling node that links ER stress and inflammation. TXNIP is induced by ER stress through the PERK and IRE1 pathways, induces IL-1β mRNA transcription, activates IL-1β production by the NLRP3 inflammasome, and mediates ER stress-mediated β cell death. Collectively, our results suggest that TXNIP is a potential therapeutic target for diabetes and ER stress-related human diseases such as Wolfram syndrome.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

Comment in
-
TXNIP switches tracks toward a terminal UPR.Cell Metab. 2012 Aug 8;16(2):135-7. doi: 10.1016/j.cmet.2012.07.012. Cell Metab. 2012. PMID: 22883225
Similar articles
-
IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress.Cell Metab. 2012 Aug 8;16(2):250-64. doi: 10.1016/j.cmet.2012.07.007. Cell Metab. 2012. PMID: 22883233 Free PMC article.
-
Unfolded protein response-activated NLRP3 inflammasome contributes to pyroptotic and apoptotic podocyte injury in diabetic kidney disease via the CHOP-TXNIP axis.Cell Signal. 2025 Jun;130:111702. doi: 10.1016/j.cellsig.2025.111702. Epub 2025 Feb 26. Cell Signal. 2025. PMID: 40020889
-
Endoplasmic Reticulum Stress Induces ROS Production and Activates NLRP3 Inflammasome Via the PERK-CHOP Signaling Pathway in Dry Eye Disease.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):34. doi: 10.1167/iovs.65.14.34. Invest Ophthalmol Vis Sci. 2024. PMID: 39699913 Free PMC article.
-
TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target.Exp Mol Med. 2023 Jul;55(7):1348-1356. doi: 10.1038/s12276-023-01019-8. Epub 2023 Jul 3. Exp Mol Med. 2023. PMID: 37394581 Free PMC article. Review.
-
NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases.Redox Biol. 2015;4:296-307. doi: 10.1016/j.redox.2015.01.008. Epub 2015 Jan 14. Redox Biol. 2015. PMID: 25625584 Free PMC article. Review.
Cited by
-
Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro.Acta Pharmacol Sin. 2015 Jul;36(7):821-30. doi: 10.1038/aps.2015.21. Epub 2015 May 25. Acta Pharmacol Sin. 2015. PMID: 26005910 Free PMC article.
-
SIRT3 Overexpression Attenuates Palmitate-Induced Pancreatic β-Cell Dysfunction.PLoS One. 2015 Apr 27;10(4):e0124744. doi: 10.1371/journal.pone.0124744. eCollection 2015. PLoS One. 2015. PMID: 25915406 Free PMC article.
-
Micro(RNA)managing endoplasmic reticulum stress.IUBMB Life. 2013 May;65(5):373-81. doi: 10.1002/iub.1151. Epub 2013 Apr 3. IUBMB Life. 2013. PMID: 23554021 Free PMC article. Review.
-
AIM2 inflammasome is activated by pharmacological disruption of nuclear envelope integrity.Proc Natl Acad Sci U S A. 2016 Aug 9;113(32):E4671-80. doi: 10.1073/pnas.1602419113. Epub 2016 Jul 26. Proc Natl Acad Sci U S A. 2016. PMID: 27462105 Free PMC article.
-
Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke.J Neuroinflammation. 2021 May 31;18(1):123. doi: 10.1186/s12974-021-02137-8. J Neuroinflammation. 2021. PMID: 34059091 Free PMC article. Review.
References
-
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Casas S, Gomis R, Gribble FM, Altirriba J, Knuutila S, Novials A. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes. 2007;56:2284–2294. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK042394/DK/NIDDK NIH HHS/United States
- R37 DK016746/DK/NIDDK NIH HHS/United States
- R24 DK093074/DK/NIDDK NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- DK016746/DK/NIDDK NIH HHS/United States
- R01 DK067493/DK/NIDDK NIH HHS/United States
- R01 DK080339/DK/NIDDK NIH HHS/United States
- RR024992/RR/NCRR NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- R01 DK016746/DK/NIDDK NIH HHS/United States
- DK93074/DK/NIDDK NIH HHS/United States
- DK067493/DK/NIDDK NIH HHS/United States
- R01 DK088227/DK/NIDDK NIH HHS/United States
- R56 DK080339/DK/NIDDK NIH HHS/United States
- P01 HL057346/HL/NHLBI NIH HHS/United States
- DK088227/DK/NIDDK NIH HHS/United States
- DK080339/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P60 DK020579/DK/NIDDK NIH HHS/United States
- R01 DK042394/DK/NIDDK NIH HHS/United States
- HL052172/HL/NHLBI NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

