Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice
- PMID: 22883314
- PMCID: PMC3548985
- DOI: 10.1017/S0007114512003364
Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice
Abstract
Dietary intake of linoleic acid (LA) has increased dramatically during the twentieth century and is associated with a greater prevalence of obesity. Vegetable oils are recognised as suitable alternatives to fish oil (FO) in feed for Atlantic salmon (Salmo salar L.) but introduce high amounts of LA in the salmon fillet. The effect on fish consumers of such a replacement remains to be elucidated. Here, we investigate the effect of excessive dietary LA from soyabean oil (SO) on endocannabinoid levels in Atlantic salmon and mice, and study the metabolic effects in mice when SO replaces FO in feed for Atlantic salmon. Atlantic salmon were fed FO and SO for 6 months, and the salmon fillet was used to produce feed for mice. Male C57BL/6J mice were fed diets of 35% of energy as fat based on FO- and SO-enriched salmon for 16 weeks. We found that replacing FO with SO in feed for Atlantic salmon increased LA, arachidonic acid (AA), decreased EPA and DHA, elevated the endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide (AEA), and increased TAG accumulation in the salmon liver. In mice, the SO salmon diet increased LA and AA and decreased EPA and DHA in the liver and erythrocyte phospholipids, and elevated 2-AG and AEA associated with increased feed efficiency, weight gain and adipose tissue inflammation compared with mice fed the FO salmon diet. In conclusion, excessive dietary LA elevates endocannabinoids in the liver of salmon and mice, and increases weight gain and counteracts the anti-inflammatory properties of EPA and DHA in mice.
Figures
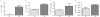
 ) and fish oil (FO,
) and fish oil (FO,
 ). Values are means, with their standard errors represented by vertical bars. *Mean values were significantly different from those of SO-fed salmon (P<0.05).
). Values are means, with their standard errors represented by vertical bars. *Mean values were significantly different from those of SO-fed salmon (P<0.05).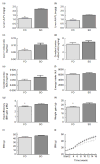
 ) and soyabean oil (SO,
) and soyabean oil (SO,
 ) salmon diets. Values are means, with their standard errors represented by vertical bars (n 8–9). *Mean values were significantly different from those of SO salmon-fed mice (P<0 05). BW, body weight.
) salmon diets. Values are means, with their standard errors represented by vertical bars (n 8–9). *Mean values were significantly different from those of SO salmon-fed mice (P<0 05). BW, body weight.
Similar articles
-
Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet.Lipids. 2014 Jan;49(1):59-69. doi: 10.1007/s11745-013-3842-y. Epub 2013 Oct 1. Lipids. 2014. PMID: 24081493 Free PMC article.
-
Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity.Obesity (Silver Spring). 2012 Oct;20(10):1984-94. doi: 10.1038/oby.2012.38. Epub 2012 Feb 15. Obesity (Silver Spring). 2012. PMID: 22334255 Free PMC article.
-
Intake of farmed Atlantic salmon fed soybean oil increases hepatic levels of arachidonic acid-derived oxylipins and ceramides in mice.J Nutr Biochem. 2015 Jun;26(6):585-95. doi: 10.1016/j.jnutbio.2014.12.005. Epub 2015 Feb 4. J Nutr Biochem. 2015. PMID: 25776459
-
Intake of farmed Atlantic salmon fed soybean oil increases insulin resistance and hepatic lipid accumulation in mice.PLoS One. 2013;8(1):e53094. doi: 10.1371/journal.pone.0053094. Epub 2013 Jan 2. PLoS One. 2013. PMID: 23301026 Free PMC article.
-
Are we what we eat? Changes to the feed fatty acid composition of farmed salmon and its effects through the food chain.J Exp Biol. 2018 Mar 7;221(Pt Suppl 1):jeb161521. doi: 10.1242/jeb.161521. J Exp Biol. 2018. PMID: 29514891 Review.
Cited by
-
Effect of flaxseed oil supplementation on the erythrocyte membrane fatty acid composition and endocannabinoid system modulation in patients with coronary artery disease: a double-blind randomized controlled trial.Genes Nutr. 2020 May 5;15(1):9. doi: 10.1186/s12263-020-00665-1. Genes Nutr. 2020. PMID: 32370762 Free PMC article.
-
Low levels of very-long-chain n-3 PUFA in Atlantic salmon (Salmo salar) diet reduce fish robustness under challenging conditions in sea cages.J Nutr Sci. 2017 Jun 28;6:e32. doi: 10.1017/jns.2017.28. eCollection 2017. J Nutr Sci. 2017. PMID: 29152236 Free PMC article.
-
Effect of a High Linoleic Acid Diet on Pregnant Women and Their Offspring.Nutrients. 2024 Sep 6;16(17):3019. doi: 10.3390/nu16173019. Nutrients. 2024. PMID: 39275331 Free PMC article. Review.
-
Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet.Lipids. 2014 Jan;49(1):59-69. doi: 10.1007/s11745-013-3842-y. Epub 2013 Oct 1. Lipids. 2014. PMID: 24081493 Free PMC article.
-
Positional Distribution of Fatty Acids in Triacylglycerols and Phospholipids from Fillets of Atlantic Salmon (Salmo Salar) Fed Vegetable and Fish Oil Blends.Mar Drugs. 2015 Jul 10;13(7):4255-69. doi: 10.3390/md13074255. Mar Drugs. 2015. PMID: 26184234 Free PMC article.
References
-
- Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. - PubMed
-
- Lands WE, Libelt B, Morris A, et al. Maintenance of lower proportions of (n-6) eicosanoid precursors in phospholipids of human plasma in response to added dietary (n-3) fatty acids. Biochim Biophys Acta. 1992;1180:147–162. - PubMed
-
- Artmann A, Petersen G, Hellgren LI, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim Biophys Acta. 2008;1781:200–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

