Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial
- PMID: 22883507
- PMCID: PMC3774022
- DOI: 10.1016/S0140-6736(12)61190-8
Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial
Abstract
Background: In view of evidence that statin therapy increases risk of diabetes, the balance of benefit and risk of these drugs in primary prevention has become controversial. We undertook an analysis of participants from the JUPITER trial to address the balance of vascular benefits and diabetes hazard of statin use.
Methods: In the randomised, double-blind JUPITER trial, 17,603 men and women without previous cardiovascular disease or diabetes were randomly assigned to rosuvastatin 20 mg or placebo and followed up for up to 5 years for the primary endpoint (myocardial infarction, stroke, admission to hospital for unstable angina, arterial revascularisation, or cardiovascular death) and the protocol-prespecified secondary endpoints of venous thromboembolism, all-cause mortality, and incident physician-reported diabetes. In this analysis, participants were stratified on the basis of having none or at least one of four major risk factors for developing diabetes: metabolic syndrome, impaired fasting glucose, body-mass index 30 kg/m(2) or higher, or glycated haemoglobin A(1c) greater than 6%. The trial is registered at ClinicalTrials.gov, NCT00239681.
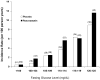
Findings: Trial participants with one or more major diabetes risk factor (n=11,508) were at higher risk of developing diabetes than were those without a major risk factor (n=6095). In individuals with one or more risk factors, statin allocation was associated with a 39% reduction in the primary endpoint (hazard ratio [HR] 0·61, 95% CI 0·47-0·79, p=0·0001), a 36% reduction in venous thromboembolism (0·64, 0·39-1·06, p=0·08), a 17% reduction in total mortality (0·83, 0·64-1·07, p=0·15), and a 28% increase in diabetes (1·28, 1·07-1·54, p=0·01). Thus, for those with diabetes risk factors, a total of 134 vascular events or deaths were avoided for every 54 new cases of diabetes diagnosed. For trial participants with no major diabetes risk factors, statin allocation was associated with a 52% reduction in the primary endpoint (HR 0·48, 95% CI 0·33-0·68, p=0·0001), a 53% reduction in venous thromboembolism (0·47, 0·21-1·03, p=0·05), a 22% reduction in total mortality (0·78, 0·59-1·03, p=0·08), and no increase in diabetes (0·99, 0·45-2·21, p=0·99). For such individuals, a total of 86 vascular events or deaths were avoided with no new cases of diabetes diagnosed. In analysis limited to the 486 participants who developed diabetes during follow-up (270 on rosuvastatin vs 216 on placebo; HR 1·25, 95% CI 1·05-1·49, p=0·01), the point estimate of cardiovascular risk reduction associated with statin therapy (HR 0·63, 95% CI 0·25-1·60) was consistent with that for the trial as a whole (0·56, 0·46-0·69). By comparison with placebo, statins accelerated the average time to diagnosis of diabetes by 5·4 weeks (84·3 [SD 47·8] weeks on rosuvastatin vs 89·7 [50·4] weeks on placebo).
Interpretation: In the JUPITER primary prevention trial, the cardiovascular and mortality benefits of statin therapy exceed the diabetes hazard, including in participants at high risk of developing diabetes.
Funding: AstraZeneca.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
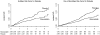

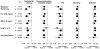
Comment in
-
Balancing the cardiometabolic benefits and risks of statins.Lancet. 2012 Aug 11;380(9841):541-3. doi: 10.1016/S0140-6736(12)61301-4. Lancet. 2012. PMID: 22883491 No abstract available.
-
Statins and risk of diabetes mellitus.Nat Rev Cardiol. 2012 Oct;9(10):552. doi: 10.1038/nrcardio.2012.126. Epub 2012 Aug 28. Nat Rev Cardiol. 2012. PMID: 22922594 No abstract available.
-
Statins induce hyperglycaemia of uncertain importance.Evid Based Med. 2013 Aug;18(4):157-8. doi: 10.1136/eb-2012-101030. Epub 2012 Nov 2. Evid Based Med. 2013. PMID: 23125233 No abstract available.
References
-
- Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. - PubMed
-
- Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. - PubMed
-
- Preiss D, Seshasai SRK, Wels P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy. JAMA. 2011;305:2556–64. - PubMed
-
- United States Food and Drug Administration. [accessed March 4, 2012];FDA Drug Safety Communication: Important safety label changes to cholesterol lowering statin drugs. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

