Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: implications for reactive astrogliosis and neurodegeneration
- PMID: 22884085
- PMCID: PMC3488579
- DOI: 10.1186/1742-2094-9-195
Astrocyte elevated gene-1 regulates astrocyte responses to neural injury: implications for reactive astrogliosis and neurodegeneration
Abstract
Background: Reactive astrogliosis is a ubiquitous but poorly understood hallmark of central nervous system pathologies such as trauma and neurodegenerative diseases. In vitro and in vivo studies have identified proinflammatory cytokines and chemokines as mediators of astrogliosis during injury and disease; however, the molecular mechanism remains unclear. In this study, we identify astrocyte elevated gene-1 (AEG-1), a human immunodeficiency virus 1 or tumor necrosis factor α-inducible oncogene, as a novel modulator of reactive astrogliosis. AEG-1 has engendered tremendous interest in the field of cancer research as a therapeutic target for aggressive tumors. However, little is known of its role in astrocytes and astrocyte-mediated diseases. Based on its oncogenic role in several cancers, here we investigate the AEG-1-mediated regulation of astrocyte migration and proliferation during reactive astrogliosis.
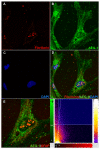
Methods: An in vivo brain injury mouse model was utilized to show AEG-1 induction following reactive astrogliosis. In vitro wound healing and cell migration assays following AEG-1 knockdown were performed to analyze the role of AEG-1 in astrocyte migration. AEG-1-mediated regulation of astrocyte proliferation was assayed by quantifying the levels of cell proliferation markers, Ki67 and proliferation cell nuclear antigen, using immunocytochemistry. Confocal microscopy was used to evaluate nucleolar localization of AEG-1 in cultured astrocytes following injury.
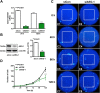
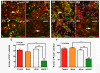
Results: The in vivo mouse model for brain injury showed reactive astrocytes with increased glial fibrillary acidic protein and AEG-1 colocalization at the wound site. AEG-1 knockdown in cultured human astrocytes significantly reduced astrocyte migration into the wound site and cell proliferation. Confocal analysis showed colocalization of AEG-1 to the nucleolus of injured cultured human astrocytes.
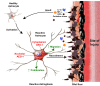
Conclusions: The present findings report for the first time the novel role of AEG-1 in mediating reactive astrogliosis and in regulating astrocyte responses to injury. We also report the nucleolar localization of AEG-1 in human astrocytes in response to injury. Future studies may be directed towards elucidating the molecular mechanism of AEG-1 action in astrocytes during reactive astrogliosis.
Figures


Similar articles
-
Reactive astrogliosis induced by TNF-α is associated with upregulated AEG-1 together with activated NF-κB pathway in vitro.Neurosci Lett. 2024 Aug 10;837:137899. doi: 10.1016/j.neulet.2024.137899. Epub 2024 Jul 15. Neurosci Lett. 2024. PMID: 39019146
-
Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury.J Neurosci. 2020 Dec 9;40(50):9751-9771. doi: 10.1523/JNEUROSCI.0888-20.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158962 Free PMC article.
-
The metabesity factor HMG20A potentiates astrocyte survival and reactive astrogliosis preserving neuronal integrity.Theranostics. 2021 May 12;11(14):6983-7004. doi: 10.7150/thno.57237. eCollection 2021. Theranostics. 2021. PMID: 34093866 Free PMC article.
-
Astrocyte elevated gene-1 (AEG-1) and the A(E)Ging HIV/AIDS-HAND.Prog Neurobiol. 2017 Oct;157:133-157. doi: 10.1016/j.pneurobio.2016.03.006. Epub 2016 Apr 14. Prog Neurobiol. 2017. PMID: 27090750 Free PMC article. Review.
-
Astrocyte elevated gene-1: recent insights into a novel gene involved in tumor progression, metastasis and neurodegeneration.Pharmacol Ther. 2007 May;114(2):155-70. doi: 10.1016/j.pharmthera.2007.01.010. Epub 2007 Feb 24. Pharmacol Ther. 2007. PMID: 17397930 Free PMC article. Review.
Cited by
-
Neurological Impact of Respiratory Viruses: Insights into Glial Cell Responses in the Central Nervous System.Microorganisms. 2024 Aug 20;12(8):1713. doi: 10.3390/microorganisms12081713. Microorganisms. 2024. PMID: 39203555 Free PMC article. Review.
-
The role of AEG-1 in the development of liver cancer.Hepat Oncol. 2015;2(3):303-312. doi: 10.2217/hep.15.10. Hepat Oncol. 2015. PMID: 26798451 Free PMC article.
-
Cytotoxic Effects of Environmental Toxins on Human Glial Cells.Neurotox Res. 2017 Feb;31(2):245-258. doi: 10.1007/s12640-016-9678-5. Epub 2016 Oct 29. Neurotox Res. 2017. PMID: 27796937
-
Metadherin facilitates podocyte apoptosis in diabetic nephropathy.Cell Death Dis. 2016 Nov 24;7(11):e2477. doi: 10.1038/cddis.2016.335. Cell Death Dis. 2016. PMID: 27882943 Free PMC article.
-
Sitagliptin attenuates high glucose-induced alterations in migration, proliferation, calcification and apoptosis of vascular smooth muscle cells through ERK1/2 signal pathway.Oncotarget. 2017 Aug 24;8(44):77168-77180. doi: 10.18632/oncotarget.20417. eCollection 2017 Sep 29. Oncotarget. 2017. PMID: 29100378 Free PMC article.
References
-
- Sun W, Fan YZ, Xi H, Lu XS, Ye C, Zhang JT. Astrocyte elevated gene-1 overexpression in human primary gallbladder carcinomas: an unfavorable and independent prognostic factor. Oncol Rep. 2011;26:1133–1142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

