Cortical metabolism in pyruvate dehydrogenase deficiency revealed by ex vivo multiplet (13)C NMR of the adult mouse brain
- PMID: 22884585
- PMCID: PMC3816779
- DOI: 10.1016/j.neuint.2012.07.020
Cortical metabolism in pyruvate dehydrogenase deficiency revealed by ex vivo multiplet (13)C NMR of the adult mouse brain
Abstract
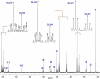
The pyruvate dehydrogenase complex (PDC), required for complete glucose oxidation, is essential for brain development. Although PDC deficiency is associated with a severe clinical syndrome, little is known about its effects on either substrate oxidation or synthesis of key metabolites such as glutamate and glutamine. Computational simulations of brain metabolism indicated that a 25% reduction in flux through PDC and a corresponding increase in flux from an alternative source of acetyl-CoA would substantially alter the (13)C NMR spectrum obtained from brain tissue. Therefore, we evaluated metabolism of [1,6-(13)C(2)]glucose (oxidized by both neurons and glia) and [1,2-(13)C(2)]acetate (an energy source that bypasses PDC) in the cerebral cortex of adult mice mildly and selectively deficient in brain PDC activity, a viable model that recapitulates the human disorder. Intravenous infusions were performed in conscious mice and extracts of brain tissue were studied by (13)C NMR. We hypothesized that mice deficient in PDC must increase the proportion of energy derived from acetate metabolism in the brain. Unexpectedly, the distribution of (13)C in glutamate and glutamine, a measure of the relative flux of acetate and glucose into the citric acid cycle, was not altered. The (13)C labeling pattern in glutamate differed significantly from glutamine, indicating preferential oxidation of [1,2-(13)C]acetate relative to [1,6-(13)C]glucose by a readily discernible metabolic domain of the brain of both normal and mutant mice, presumably glia. These findings illustrate that metabolic compartmentation is preserved in the PDC-deficient cerebral cortex, probably reflecting intact neuron-glia metabolic interactions, and that a reduction in brain PDC activity sufficient to induce cerebral dysgenesis during development does not appreciably disrupt energy metabolism in the mature brain.
Copyright © 2012. Published by Elsevier Ltd.
Figures




References
-
- Blin M, Crusio WE, Hevor T, Cloix JF. Chronic inhibition of glutamine synthetase is not associated with impairment of learning and memory in mice. Brain Res Bull. 2002;57:11–15. - PubMed
-
- Cardell M, Koide T, Wieloch T. Pyruvate dehydrogenase activity in the rat cerebral cortex following cerebral ischemia. J Cereb Blood Flow Metab. 1989;9:350–357. - PubMed
-
- Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in vivo and in vitro 13C NMR. J Biol Chem. 1990;265:12916–12926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

