At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response
- PMID: 22884900
- PMCID: PMC4157329
- DOI: 10.1016/j.bbi.2012.07.012
At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response
Abstract
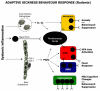
Delirium is a common and severe neuropsychiatric syndrome characterised by acute deterioration and fluctuations in mental status. It is precipitated mainly by acute illness, trauma, surgery, or drugs. Delirium affects around one in eight hospital inpatients and is associated with multiple adverse consequences, including new institutionalisation, worsening of existing dementia, and death. Patients with delirium show attentional and other cognitive deficits, altered alertness (mostly reduced, but some patients develop agitation and hyperactivity), altered sleep-wake cycle and psychoses. The pathways from the various aetiologies to the heterogeneous clinical presentations are hardly studied and are poorly understood. One of the key questions, which research is only now beginning to address, is how the factors determining susceptibility interact with the stimuli that trigger delirium. Inflammatory signals arising during systemic infection evoke sickness behaviour, a coordinated set of adaptive changes initiated by the host to respond to, and to counteract, infection. It is now clear that the same systemic inflammatory signals can have severe deleterious effects on brain function when occuring in old age or in the presence of neurodegenerative disease. Multiple animal studies now show that even mild acute systemic inflammation can induce exaggerated sickness behaviour responses and cognitive dysfunction in aged animals or those with prior degenerative pathology when compared to young and/or healthy controls. These findings appear highly promising in understanding aspects of delirium. In this review our aim is to describe and assess the parallels between exaggerated sickness behaviour in vulnerable animals and delirium in older humans. We discuss inflammatory and stress-related triggers of delirium in the context of new animal models that allow us to dissect some aspects of the mechanisms underpinning these episodes. We discuss some differences between the sickness behaviour syndrome model and delirium in the context of the complexity in the latter due to other factors such as prior pathology, psychological stress and drug effects. We conclude that, with appropriate caveats, the study of sickness behaviour in the vulnerable brain offers a promising route to uncover the mechanisms of this common and serious unmet medical need.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Bahagon Y, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM. Prevalence and predictive features of bacteremic urinary tract infection in emergency department patients. Eur J Clin Microbiol Infect Dis. 2007;26:349–352. - PubMed
-
- Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. - PubMed
-
- Barichello T, Martins MR, Reinke A, Feier G, Ritter C, Quevedo J, Dal-Pizzol F. Long-term cognitive impairment in sepsis survivors. Crit Care Med. 2005;33:1671. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

