Drosulfakinin activates CCKLR-17D1 and promotes larval locomotion and escape response in Drosophila
- PMID: 22885328
- PMCID: PMC3519664
- DOI: 10.4161/fly.21534
Drosulfakinin activates CCKLR-17D1 and promotes larval locomotion and escape response in Drosophila
Abstract
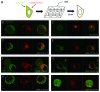
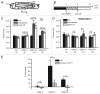
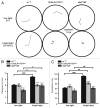
Neuropeptides are ubiquitous in both mammals and invertebrates and play essential roles in regulation and modulation of many developmental and physiological processes through activation of G-protein-coupled-receptors (GPCRs). However, the mechanisms by which many of the neuropeptides regulate specific neural function and behaviors remain undefined. Here we investigate the functions of Drosulfakinin (DSK), the Drosophila homolog of vertebrate neuropeptide cholecystokinin (CCK), which is the most abundant neuropeptide in the central nervous system. We provide biochemical evidence that sulfated DSK-1 and DSK-2 activate the CCKLR-17D1 receptor in a cell culture assay. We further examine the role of DSK and CCKLR-17D1 in the regulation of larval locomotion, both in a semi-intact larval preparation and in intact larvae under intense light exposure. Our results suggest that DSK/CCKLR-17D1 signaling promote larval body wall muscle contraction and is necessary for mediating locomotor behavior in stress-induced escape response.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
