Effects of bariatric surgery on glucose homeostasis and type 2 diabetes
- PMID: 22885332
- PMCID: PMC3462491
- DOI: 10.1053/j.gastro.2012.07.114
Effects of bariatric surgery on glucose homeostasis and type 2 diabetes
Abstract
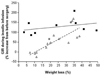
Obesity is an important risk factor for type 2 diabetes mellitus (T2DM). Weight loss improves the major factors involved in the pathogenesis of T2DM, namely insulin action and beta cell function, and is considered a primary therapy for obese patients who have T2DM. Unfortunately, most patients with T2DM fail to achieve successful weight loss and adequate glycemic control from medical therapy. In contrast, bariatric surgery causes marked weight loss and complete remission of T2DM in most patients. Moreover, bariatric surgical procedures that divert nutrients away from the upper gastrointestinal tract are more successful in producing weight loss and remission of T2DM than those that simply restrict stomach capacity. Although upper gastrointestinal tract bypass procedures alter the metabolic response to meal ingestion, by increasing early postprandial plasma concentrations of glucagon-like peptide 1 and insulin, it is not clear whether these effects make an important contribution to long-term control of glycemia and T2DM once substantial surgery-induced weight loss has occurred. Nonetheless, the effects of surgery on body weight and metabolic function indicate that bariatric surgery should be part of the standard therapy for T2DM. More research is needed to advance our understanding of the physiological effects of different bariatric surgical procedures and possible weight loss-independent factors that improve metabolic function and contribute to the resolution of T2DM.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. - PubMed
-
- Chan JM, Rimm EB, Colditz GA, et al. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. - PubMed
-
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. - PubMed
-
- Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. - PubMed
-
- DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999;131:281–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

