Hedgehog controls hepatic stellate cell fate by regulating metabolism
- PMID: 22885334
- PMCID: PMC3480563
- DOI: 10.1053/j.gastro.2012.07.115
Hedgehog controls hepatic stellate cell fate by regulating metabolism
Abstract
Background & aims: The pathogenesis of cirrhosis, a disabling outcome of defective liver repair, involves deregulated accumulation of myofibroblasts derived from quiescent hepatic stellate cells (HSCs), but the mechanisms that control transdifferentiation of HSCs are poorly understood. We investigated whether the Hedgehog (Hh) pathway controls the fate of HSCs by regulating metabolism.
Methods: Microarray, quantitative polymerase chain reaction, and immunoblot analyses were used to identify metabolic genes that were differentially expressed in quiescent vs myofibroblast HSCs. Glycolysis and lactate production were disrupted in HSCs to determine if metabolism influenced transdifferentiation. Hh signaling and hypoxia-inducible factor 1α (HIF1α) activity were altered to identify factors that alter glycolytic activity. Changes in expression of genes that regulate glycolysis were quantified and localized in biopsy samples from patients with cirrhosis and liver samples from mice following administration of CCl(4) or bile duct ligation. Mice were given systemic inhibitors of Hh to determine if they affect glycolytic activity of the hepatic stroma; Hh signaling was also conditionally disrupted in myofibroblasts to determine the effects of glycolytic activity.
Results: Transdifferentiation of cultured, quiescent HSCs into myofibroblasts induced glycolysis and caused lactate accumulation. Increased expression of genes that regulate glycolysis required Hh signaling and involved induction of HIF1α. Inhibitors of Hh signaling, HIF1α, glycolysis, or lactate accumulation converted myofibroblasts to quiescent HSCs. In diseased livers of animals and patients, numbers of glycolytic stromal cells were associated with the severity of fibrosis. Conditional disruption of Hh signaling in myofibroblasts reduced numbers of glycolytic myofibroblasts and liver fibrosis in mice; similar effects were observed following administration of pharmacologic inhibitors of Hh.
Conclusions: Hedgehog signaling controls the fate of HSCs by regulating metabolism. These findings might be applied to diagnosis and treatment of patients with cirrhosis.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

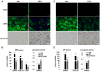
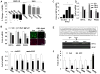



Similar articles
-
Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells.Gastroenterology. 2018 Apr;154(5):1465-1479.e13. doi: 10.1053/j.gastro.2017.12.022. Epub 2018 Jan 3. Gastroenterology. 2018. PMID: 29305935 Free PMC article.
-
Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis.Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297(6):G1093-106. doi: 10.1152/ajpgi.00292.2009. Epub 2009 Oct 8. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19815628 Free PMC article.
-
Gli2-regulated activation of hepatic stellate cells and liver fibrosis by TGF-β signaling.Am J Physiol Gastrointest Liver Physiol. 2021 May 1;320(5):G720-G728. doi: 10.1152/ajpgi.00310.2020. Epub 2021 Mar 17. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33728992
-
Mitochondria: A critical hub for hepatic stellate cells activation during chronic liver diseases.Hepatobiliary Pancreat Dis Int. 2021 Aug;20(4):315-322. doi: 10.1016/j.hbpd.2021.04.010. Epub 2021 Apr 28. Hepatobiliary Pancreat Dis Int. 2021. PMID: 33975780 Review.
-
MicroRNAs in liver fibrosis: Focusing on the interaction with hedgehog signaling.World J Gastroenterol. 2016 Aug 7;22(29):6652-62. doi: 10.3748/wjg.v22.i29.6652. World J Gastroenterol. 2016. PMID: 27547008 Free PMC article. Review.
Cited by
-
Role of the Hippo pathway in liver regeneration and repair: recent advances.Inflamm Regen. 2022 Dec 5;42(1):59. doi: 10.1186/s41232-022-00235-5. Inflamm Regen. 2022. PMID: 36471376 Free PMC article. Review.
-
Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation.Circ Res. 2020 Jul 17;127(3):427-447. doi: 10.1161/CIRCRESAHA.120.316958. Epub 2020 Jul 16. Circ Res. 2020. PMID: 32673537 Free PMC article. Review.
-
Metabolic Signature of Hepatic Fibrosis: From Individual Pathways to Systems Biology.Cells. 2019 Nov 12;8(11):1423. doi: 10.3390/cells8111423. Cells. 2019. PMID: 31726658 Free PMC article. Review.
-
Fibrogenic Pathways in Metabolic Dysfunction Associated Fatty Liver Disease (MAFLD).Int J Mol Sci. 2022 Jun 23;23(13):6996. doi: 10.3390/ijms23136996. Int J Mol Sci. 2022. PMID: 35805998 Free PMC article. Review.
-
Interleukin-1β attenuates myofibroblast formation and extracellular matrix production in dermal and lung fibroblasts exposed to transforming growth factor-β1.PLoS One. 2014 Mar 12;9(3):e91559. doi: 10.1371/journal.pone.0091559. eCollection 2014. PLoS One. 2014. PMID: 24622053 Free PMC article.
References
-
- Tsukamoto H. Adipogenic phenotype of hepatic stellate cells. Alcohol Clin Exp Res. 2005;29:132S–133S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

