zRICH, a protein induced during optic nerve regeneration in zebrafish, promotes neuritogenesis and interacts with tubulin
- PMID: 22885342
- PMCID: PMC3526659
- DOI: 10.1016/j.brainres.2012.07.057
zRICH, a protein induced during optic nerve regeneration in zebrafish, promotes neuritogenesis and interacts with tubulin
Abstract
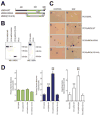
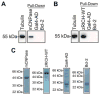
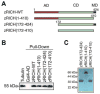
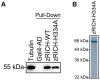
Mammals do not regenerate axons in their central nervous system (CNS) spontaneously. This phenomenon is the cause of numerous medical conditions after damage to nerve fibers in the CNS of humans. The study of the mechanisms of nerve regeneration in other vertebrate animals able to spontaneously regenerate axons in their CNS is essential for understanding nerve regeneration from a scientific point of view, and for developing therapeutic approaches to enhance nerve regeneration in the CNS of humans. RICH proteins are a novel group of proteins implicated in nerve regeneration in the CNS of teleost fish, yet their mechanisms of action are not well understood. A number of mutant versions of the zebrafish RICH (zRICH) protein were generated and characterized at biochemical and cellular levels in our laboratory. With the aim of understanding the effects of RICH proteins in neuronal axon outgrowth, stable transfectants derived from the neuronal model PC12 cell line expressing zRICH Wild-Type or mutant versions of zRICH were studied. Results from differentiation experiments suggest that RICH proteins enhance neuronal plasticity by facilitating neurite branching. Biochemical co-purification results have demonstrated that zRICH binds to the cytoskeletal protein tubulin. The central domain of the protein is sufficient for tubulin binding, but a mutant version of the protein lacking the terminal domains, which cannot bind to the plasma membrane, was not able to enhance neurite branching. RICH proteins may facilitate axon regeneration by regulating the axonal cytoskeleton and facilitating the formation of new neurite branches.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Enhancing structural plasticity of PC12 neurons during differentiation and neurite regeneration with a catalytically inactive mutant version of the zRICH protein.BMC Neurosci. 2023 Aug 23;24(1):43. doi: 10.1186/s12868-023-00808-1. BMC Neurosci. 2023. PMID: 37612637 Free PMC article.
-
Characterization of the domains of zRICH, a protein induced during optic nerve regeneration in zebrafish.Brain Res. 2006 Jul 19;1100(1):42-54. doi: 10.1016/j.brainres.2006.04.123. Epub 2006 Jun 12. Brain Res. 2006. PMID: 16765331
-
Cloning and characterization of zRICH, a 2',3'-cyclic-nucleotide 3'-phosphodiesterase induced during zebrafish optic nerve regeneration.J Neurochem. 1999 Apr;72(4):1362-71. doi: 10.1046/j.1471-4159.1999.721362.x. J Neurochem. 1999. PMID: 10098837
-
The Regulatory Role of Reticulons in Neurodegeneration: Insights Underpinning Therapeutic Potential for Neurodegenerative Diseases.Cell Mol Neurobiol. 2021 Aug;41(6):1157-1174. doi: 10.1007/s10571-020-00893-4. Epub 2020 Jun 5. Cell Mol Neurobiol. 2021. PMID: 32504327 Free PMC article. Review.
-
Zebrafish as a translational regeneration model to study the activation of neural stem cells and role of their environment.Rev Neurosci. 2018 Dec 19;30(1):45-66. doi: 10.1515/revneuro-2018-0020. Rev Neurosci. 2018. PMID: 30067512 Review.
Cited by
-
Enhancing structural plasticity of PC12 neurons during differentiation and neurite regeneration with a catalytically inactive mutant version of the zRICH protein.BMC Neurosci. 2023 Aug 23;24(1):43. doi: 10.1186/s12868-023-00808-1. BMC Neurosci. 2023. PMID: 37612637 Free PMC article.
References
-
- Agranoff BW, Ford-Holevinski TS. Biochemical aspects of the regenerating goldfish visual system. In: Elam JS, Cancalon P, editors. Axonal transport in neuronal growth and regeneration. Plenum Press; New York: 1984. pp. 69–86.
-
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
-
- Asch WS, Leake D, Canger AK, Passini MA, Argenton F, Schechter N. Cloning of zebrafish neurofilament cDNAs for plasticin and gefiltin: increased mRNA expression in ganglion cells after optic nerve injury. J Neurochem. 1998;71:20–32. - PubMed
-
- Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins - promising targets for protection in neurodegenerative diseases. Biol Chem. 2010;391:619–629. - PubMed
-
- Bahr M, Bonhoeffer F. Perspectives on axonal regeneration in the mammalian CNS. Trends Neurosci. 1994;17:473–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

