Bile acid transporters and regulatory nuclear receptors in the liver and beyond
- PMID: 22885388
- PMCID: PMC3526785
- DOI: 10.1016/j.jhep.2012.08.002
Bile acid transporters and regulatory nuclear receptors in the liver and beyond
Abstract
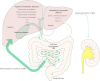
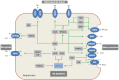
Bile acid (BA) transporters are critical for maintenance of the enterohepatic BA circulation where BAs exert their multiple physiological functions including stimulation of bile flow, intestinal absorption of lipophilic nutrients, solubilization and excretion of cholesterol, as well as antimicrobial and metabolic effects. Tight regulation of BA transporters via nuclear receptors is necessary to maintain proper BA homeostasis. Hereditary and acquired defects of BA transporters are involved in the pathogenesis of several hepatobiliary disorders including cholestasis, gallstones, fatty liver disease and liver cancer, but also play a role in intestinal and metabolic disorders beyond the liver. Thus, pharmacological modification of BA transporters and their regulatory nuclear receptors opens novel treatment strategies for a wide range of disorders.
Copyright © 2012 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Hofmann A.F. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. - PubMed
-
- Hofmann A.F. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. - PubMed
-
- Trauner M., Boyer J.L. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83:633–671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

