Insights into the neurobiology of the nicotinic cholinergic system and nicotine addiction from mice expressing nicotinic receptors harboring gain-of-function mutations
- PMID: 22885704
- PMCID: PMC3462994
- DOI: 10.1124/pr.111.004671
Insights into the neurobiology of the nicotinic cholinergic system and nicotine addiction from mice expressing nicotinic receptors harboring gain-of-function mutations
Abstract
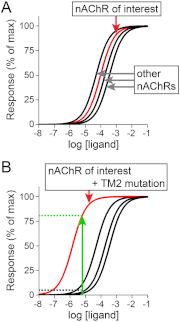
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated, cation-selective ion channels expressed throughout the brain. Although these channels have been investigated for several decades, it is still challenging 1) to identify the important nAChR subunits in cholinergic transmission and nicotine dependence and 2) to develop nAChR subtype-specific ligands. To overcome these challenges, we and others have studied mice expressing mutant, gain-of-function nAChR subunits. In this review, we discuss this research approach and the results it has yielded to date. Gain-of-function mutations, including those in nAChR subunits, provide an approach that is complementary to loss-of-function studies such as gene knockouts; the former allows one to answer questions of sufficiency and the latter addresses questions of necessity. Mutant mice expressing gain-of-function nAChR subunits are commonly produced using traditional gene targeting in embryonic stem cells, but novel approaches such as bacterial artificial chromosome transgenesis have yielded important insights as well. α7 nAChRs were the first nAChRs to be targeted with a gain-of-function mutation, followed by a pair of α4 nAChR gain-of-function mutant mice. These α4 nAChR gain-of-function mice (α4 L9'S mice, followed by α4 L9'A mice) provided an important system to probe α4 nAChR function in vivo, particularly in the dopamine reward system. α6 nAChR gain-of-function mice provided the first robust system allowing specific manipulation of this receptor subtype. Other targeted mutations in various nAChR subunits have also been produced and have yielded important insights into nicotinic cholinergic biology. As nAChR research advances and more details associated with nAChR expression and function emerge, we expect that existing and new mouse lines expressing gain-of-function nAChR subunits will continue to provide new insights.
Figures

Similar articles
-
Cholinergic modulation of locomotion and striatal dopamine release is mediated by alpha6alpha4* nicotinic acetylcholine receptors.J Neurosci. 2010 Jul 21;30(29):9877-89. doi: 10.1523/JNEUROSCI.2056-10.2010. J Neurosci. 2010. PMID: 20660270 Free PMC article.
-
A role for α4(non-α6)* nicotinic acetylcholine receptors in motor behavior.Neuropharmacology. 2013 Oct;73:19-30. doi: 10.1016/j.neuropharm.2013.05.001. Epub 2013 May 17. Neuropharmacology. 2013. PMID: 23688922 Free PMC article.
-
Local application of drugs to study nicotinic acetylcholine receptor function in mouse brain slices.J Vis Exp. 2012 Oct 29;(68):e50034. doi: 10.3791/50034. J Vis Exp. 2012. PMID: 23128482 Free PMC article.
-
Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice.Behav Pharmacol. 2008 Sep;19(5-6):461-84. doi: 10.1097/FBP.0b013e32830c360e. Behav Pharmacol. 2008. PMID: 18690103 Free PMC article. Review.
-
Unraveling the neurobiology of nicotine dependence using genetically engineered mice.Curr Opin Neurobiol. 2013 Aug;23(4):493-9. doi: 10.1016/j.conb.2013.02.013. Epub 2013 Mar 29. Curr Opin Neurobiol. 2013. PMID: 23545467 Free PMC article. Review.
Cited by
-
α4α6β2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function.Mol Pharmacol. 2013 Sep;84(3):393-406. doi: 10.1124/mol.113.087346. Epub 2013 Jun 20. Mol Pharmacol. 2013. PMID: 23788655 Free PMC article.
-
Expression of sensitized β2 nAChR subunits in VTA neurons enhances intravenous nicotine self-administration in male rats.Neuropharmacology. 2024 Dec 15;261:110161. doi: 10.1016/j.neuropharm.2024.110161. Epub 2024 Sep 17. Neuropharmacology. 2024. PMID: 39299573
-
Epilepsy and tobacco smoking: a cross-sectional study.J Neurol. 2016 Oct;263(10):2057-64. doi: 10.1007/s00415-016-8228-7. Epub 2016 Jul 14. J Neurol. 2016. PMID: 27416858
-
β2* nAChR sensitivity modulates acquisition of cocaine self-administration in male rats.Neuropharmacology. 2024 Jun 1;250:109927. doi: 10.1016/j.neuropharm.2024.109927. Epub 2024 Mar 18. Neuropharmacology. 2024. PMID: 38508306 Free PMC article.
-
TC299423, a Novel Agonist for Nicotinic Acetylcholine Receptors.Front Pharmacol. 2017 Sep 26;8:641. doi: 10.3389/fphar.2017.00641. eCollection 2017. Front Pharmacol. 2017. PMID: 29033834 Free PMC article.
References
-
- Absalom NL, Schofield PR, Lewis TM. (2009) Pore structure of the Cys-loop ligand-gated ion channels. Neurochem Res 34:1805–1815 - PubMed
-
- Aubin HJ, Karila L, Reynaud M. (2011) Pharmacotherapy for smoking cessation: present and future. Curr Pharm Des 17:1343–1350 - PubMed
-
- Azam L, Winzer-Serhan U, Leslie FM. (2003) Co-expression of α7 and β2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119:965–977 - PubMed
-
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444:260–274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

