Discovery of a novel (+)-γ-lactamase from Bradyrhizobium japonicum USDA 6 by rational genome mining
- PMID: 22885756
- PMCID: PMC3457094
- DOI: 10.1128/AEM.01398-12
Discovery of a novel (+)-γ-lactamase from Bradyrhizobium japonicum USDA 6 by rational genome mining
Abstract
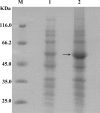
A novel (+)-γ-lactamase used for the resolution of racemic γ-lactam from Bradyrhizobium japonicum USDA 6 was found as a result of sequence-structure guided genome mining. It consists of 409 amino acids, only 49% of which are identical to the amino acid sequences of the known (+)-γ-lactamase from Sulfolobus solfataricus. This is only the third (+)-γ-lactamase gene to be reported.
Figures



Similar articles
-
Enzymatic preparation of optically pure (+)-2-azabicyclo[2.2.1]hept-5-en-3-one by (-)-γ-lactamase from Bradyrhizobium japonicum USDA 6.Bioorg Med Chem Lett. 2014 Oct 15;24(20):4899-902. doi: 10.1016/j.bmcl.2014.08.057. Epub 2014 Sep 2. Bioorg Med Chem Lett. 2014. PMID: 25240615
-
Homology modeling and docking studies of BjGL, a novel (+) gamma-lactamase from Bradyrhizobium japonicum.J Mol Graph Model. 2014 Feb;47:1-7. doi: 10.1016/j.jmgm.2013.10.006. Epub 2013 Oct 24. J Mol Graph Model. 2014. PMID: 24215997
-
Identification and characterization of a novel (+)-γ-lactamase from Microbacterium hydrocarbonoxydans.Appl Microbiol Biotechnol. 2016 Nov;100(22):9543-9553. doi: 10.1007/s00253-016-7643-0. Epub 2016 Jun 2. Appl Microbiol Biotechnol. 2016. PMID: 27255489
-
Identification of active-site residues in Bradyrhizobium japonicum malonamidase E2.Biochem J. 2000 Jul 15;349(Pt 2):501-7. doi: 10.1042/0264-6021:3490501. Biochem J. 2000. PMID: 10880349 Free PMC article.
-
[Advances in lactamases from microbes--a review].Wei Sheng Wu Xue Bao. 2010 Aug;50(8):988-94. Wei Sheng Wu Xue Bao. 2010. PMID: 20931864 Review. Chinese.
Cited by
-
Structural insights into the γ-lactamase activity and substrate enantioselectivity of an isochorismatase-like hydrolase from Microbacterium hydrocarbonoxydans.Sci Rep. 2017 Mar 15;7:44542. doi: 10.1038/srep44542. Sci Rep. 2017. PMID: 28295028 Free PMC article.
-
Construction of an organelle-like nanodevice via supramolecular self-assembly for robust biocatalysts.Microb Cell Fact. 2018 Feb 20;17(1):26. doi: 10.1186/s12934-018-0873-3. Microb Cell Fact. 2018. PMID: 29458431 Free PMC article.
-
CBD binding domain fused γ-lactamase from Sulfolobus solfataricus is an efficient catalyst for (-) γ-lactam production.BMC Biotechnol. 2014 May 13;14:40. doi: 10.1186/1472-6750-14-40. BMC Biotechnol. 2014. PMID: 24884655 Free PMC article.
-
Dynamic kinetic resolution of Vince lactam catalyzed by γ-lactamases: a mini-review.J Ind Microbiol Biotechnol. 2018 Dec;45(12):1017-1031. doi: 10.1007/s10295-018-2093-6. Epub 2018 Oct 23. J Ind Microbiol Biotechnol. 2018. PMID: 30353294 Review.
-
Engineering the Enantioselectivity and Thermostability of a (+)-γ-Lactamase from Microbacterium hydrocarbonoxydans for Kinetic Resolution of Vince Lactam (2-Azabicyclo[2.2.1]hept-5-en-3-one).Appl Environ Microbiol. 2017 Dec 15;84(1):e01780-17. doi: 10.1128/AEM.01780-17. Print 2018 Jan 1. Appl Environ Microbiol. 2017. PMID: 29054871 Free PMC article.
References
-
- Brabban AD, Littlechild J, Wisdom R. 1996. Stereospecific γ-lactamase activity in Pseudomonas fluorescens species. J. Ind. Microbiol. Biotechnol. 16:8–14
-
- Cilia E, Fabbri A, Uriani M, Scialdone GG, Ammendola S. 2005. The signature amidase from Sulfolobus solfataricus belongs to the CX3C subgroup of enzymes cleaving both amides and nitriles. FEBS J. 272:4716–4724 - PubMed
-
- Evans CT, Roberts SM, Shoberu KA, Sutherland AG. 1992. Potential use of carbocyclic nucleosides for the treatment of AIDS: chemo-enzymatic syntheses of the enantiomers of carbovir. J. Chem. Soc. Perkin Transact. 1 1:589–592
-
- Ferrer M, Golyshina O, Beloqui A, Golyshin PN. 2007. Mining enzymes from extreme environments. Curr. Opin. Microbiol. 10:207–214 - PubMed
-
- Foster RH, Faulds D. 1998. Abacavir. Drugs 55:729–738 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

