Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update
- PMID: 22885793
- PMCID: PMC3905791
- DOI: 10.1007/s00204-012-0918-z
Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update
Abstract
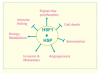
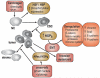
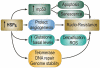
Heat shock proteins (HSP) are a subset of the molecular chaperones, best known for their rapid and abundant induction by stress. HSP genes are activated at the transcriptional level by heat shock transcription factor 1 (HSF1). During the progression of many types of cancer, this heat shock transcriptional regulon becomes co-opted by mechanisms that are currently unclear, although evidently triggered in the emerging tumor cell. Concerted activation of HSF1 and the accumulation of HSPs then participate in many of the traits that permit the malignant phenotype. Thus, cancers of many histologies exhibit activated HSF1 and increased HSP levels that may help to deter tumor suppression and evade therapy in the clinic. We review here the extensive work that has been carried out and is still in progress aimed at (1) understanding the oncogenic mechanisms by which HSP genes are switched on, (2) determining the roles of HSF1/HSP in malignant transformation and (3) discovering approaches to therapy based on disrupting the influence of the HSF1-controlled transcriptome in cancer.
Figures

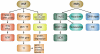
Comment in
-
Role of heat shock proteins in stress response and carcinogenesis.Arch Toxicol. 2013 Jan;87(1):1-2. doi: 10.1007/s00204-012-0985-1. Arch Toxicol. 2013. PMID: 23184253 No abstract available.
References
-
- Agarwal M, Pandita S, Hunt CR, Gupta A, Yue X, Khan S, Pandita RK, Pratt D, Shay JW, Taylor JS, Pandita TK. Inhibition of telomerase activity enhances hyperthermia-mediated radiosensitization. Cancer Res. 68:3370–3378. - PubMed
-
- Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67(2):616–625. - PubMed
-
- Ahmad MF, Raman B, Ramakrishna T, Rao Ch M. Effect of phosphorylation on alpha B-crystallin: differences in stability, subunit exchange and chaperone activity of homo and mixed oligomers of alpha B-crystallin and its phosphorylation-mimicking mutant. J Mol Biol. 2008;375(4):1040–1051. - PubMed
-
- Al-Madhoun AS, Chen YX, Haidari L, et al. The interaction and cellular localization of HSP27 and ERbeta are modulated by 17beta-estradiol and HSP27 phosphorylation. Mol Cell Endocrinol. 2007;270(1-2):33–42. - PubMed
-
- Aloy MT, Hadchity E, Bionda C, Diaz-Latoud C, Claude L, Rousson R, Arrigo AP, Rodriguez-Lafrasse C. Protective role of Hsp27 protein against gamma radiation-induced apoptosis and radiosensitization effects of Hsp27 gene silencing in different human tumor cells. Int J Radiat Oncol Biol Phys. 2008;70:543–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

