Binding and translocation of termination factor rho studied at the single-molecule level
- PMID: 22885804
- PMCID: PMC3472157
- DOI: 10.1016/j.jmb.2012.07.027
Binding and translocation of termination factor rho studied at the single-molecule level
Abstract
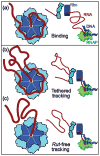
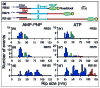
Rho termination factor is an essential hexameric helicase responsible for terminating 20-50% of all mRNA synthesis in Escherichia coli. We used single-molecule force spectroscopy to investigate Rho-RNA binding interactions at the Rho utilization site of the λtR1 terminator. Our results are consistent with Rho complexes adopting two states: one that binds 57 ± 2nt of RNA across all six of the Rho primary binding sites, and another that binds 85 ± 2nt at the six primary sites plus a single secondary site situated at the center of the hexamer. The single-molecule data serve to establish that Rho translocates 5'→3' toward RNA polymerase (RNAP) by a tethered-tracking mechanism, looping out the intervening RNA between the Rho utilization site and RNAP. These findings lead to a general model for Rho binding and translocation and establish a novel experimental approach that should facilitate additional single-molecule studies of RNA-binding proteins.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures






Comment in
-
Getting a grip on the terminator.J Mol Biol. 2012 Nov 9;423(5):661-3. doi: 10.1016/j.jmb.2012.09.004. Epub 2012 Sep 10. J Mol Biol. 2012. PMID: 22975072 No abstract available.
Similar articles
-
Regulation of rho-dependent transcription termination by NusG is specific to the Escherichia coli elongation complex.Biochemistry. 2000 May 9;39(18):5573-85. doi: 10.1021/bi992658z. Biochemistry. 2000. PMID: 10820031
-
Structural basis of the transcription termination factor Rho engagement with transcribing RNA polymerase from Thermus thermophilus.Sci Adv. 2023 Feb 10;9(6):eade7093. doi: 10.1126/sciadv.ade7093. Epub 2023 Feb 8. Sci Adv. 2023. PMID: 36753546 Free PMC article.
-
RNA secondary structures regulate three steps of Rho-dependent transcription termination within a bacterial mRNA leader.Nucleic Acids Res. 2017 Jan 25;45(2):631-642. doi: 10.1093/nar/gkw889. Epub 2016 Oct 5. Nucleic Acids Res. 2017. PMID: 28123036 Free PMC article.
-
Rho and RNA: models for recognition and response.Mol Microbiol. 1994 Mar;11(6):983-90. doi: 10.1111/j.1365-2958.1994.tb00376.x. Mol Microbiol. 1994. PMID: 8022288 Review.
-
Mechanisms of Bacterial Transcription Termination: All Good Things Must End.Annu Rev Biochem. 2016 Jun 2;85:319-47. doi: 10.1146/annurev-biochem-060815-014844. Epub 2016 Mar 17. Annu Rev Biochem. 2016. PMID: 27023849 Review.
Cited by
-
Direct observation of the translocation mechanism of transcription termination factor Rho.Nucleic Acids Res. 2015 Feb 27;43(4):2367-77. doi: 10.1093/nar/gkv085. Epub 2015 Feb 6. Nucleic Acids Res. 2015. PMID: 25662222 Free PMC article.
-
Direct observation of processive exoribonuclease motion using optical tweezers.Proc Natl Acad Sci U S A. 2015 Dec 8;112(49):15101-6. doi: 10.1073/pnas.1514028112. Epub 2015 Nov 23. Proc Natl Acad Sci U S A. 2015. PMID: 26598710 Free PMC article.
-
Real-time observation of polymerase-promoter contact remodeling during transcription initiation.Nat Commun. 2017 Oct 27;8(1):1178. doi: 10.1038/s41467-017-01041-1. Nat Commun. 2017. PMID: 29079833 Free PMC article.
-
Rho-dependent transcription termination: mechanisms and roles in bacterial fitness and adaptation to environmental changes.RNA. 2025 Aug 18;31(9):1207-1234. doi: 10.1261/rna.080486.125. RNA. 2025. PMID: 40461252 Free PMC article. Review.
-
Cryo-EM structure of transcription termination factor Rho from Mycobacterium tuberculosis reveals bicyclomycin resistance mechanism.Commun Biol. 2022 Feb 9;5(1):120. doi: 10.1038/s42003-022-03069-6. Commun Biol. 2022. PMID: 35140348 Free PMC article.
References
-
- Nudler E, Gottesman ME. Transcription termination and anti- termination in E. coli. Genes Cells. 2002;7:755–68. - PubMed
-
- Richardson JP. Rho-dependent termination and ATPases in transcript termination. Biochim Biophys Acta. 2002;1577:251–260. - PubMed
-
- Ciampi MS. Rho-dependent terminators and transcription termination. Microbiology. 2006;152:2515–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

