Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis
- PMID: 22886300
- PMCID: PMC3428082
- DOI: 10.1172/JCI61758
Dynamic T cell-APC interactions sustain chronic inflammation in atherosclerosis
Abstract
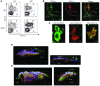
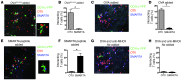
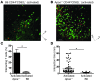
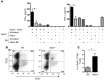
Atherosclerosis is a chronic inflammatory disease of large and medium-sized arteries characterized by leukocyte accumulation in the vessel wall. Both innate and adaptive immune responses contribute to atherogenesis, but the identity of atherosclerosis-relevant antigens and the role of antigen presentation in this disease remain poorly characterized. We developed live-cell imaging of explanted aortas to compare the behavior and role of APCs in normal and atherosclerotic mice. We found that CD4+ T cells were capable of interacting with fluorescently labeled (CD11c-YFP+) APCs in the aortic wall in the presence, but not the absence, of cognate antigen. In atherosclerosis-prone Apoe-/-CD11c-YFP+ mice, APCs extensively interacted with CD4+ T cells in the aorta, leading to cell activation and proliferation as well as secretion of IFN-γ and TNF-α. These cytokines enhanced uptake of oxidized and minimally modified LDL by macrophages. We conclude that antigen presentation by APCs to CD4+ T cells in the arterial wall causes local T cell activation and production of proinflammatory cytokines, which promote atherosclerosis by maintaining chronic inflammation and inducing foam cell formation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

