PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor
- PMID: 22886306
- PMCID: PMC3428075
- DOI: 10.1172/JCI57407
PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor
Abstract
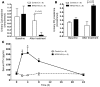
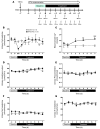
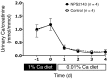
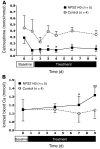
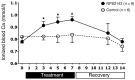
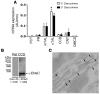
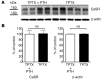
Tight regulation of calcium levels is required for many critical biological functions. The Ca2+-sensing receptor (CaSR) expressed by parathyroid cells controls blood calcium concentration by regulating parathyroid hormone (PTH) secretion. However, CaSR is also expressed in other organs, such as the kidney, but the importance of extraparathyroid CaSR in calcium metabolism remains unknown. Here, we investigated the role of extraparathyroid CaSR using thyroparathyroidectomized, PTH-supplemented rats. Chronic inhibition of CaSR selectively increased renal tubular calcium absorption and blood calcium concentration independent of PTH secretion change and without altering intestinal calcium absorption. CaSR inhibition increased blood calcium concentration in animals pretreated with a bisphosphonate, indicating that the increase did not result from release of bone calcium. Kidney CaSR was expressed primarily in the thick ascending limb of the loop of Henle (TAL). As measured by in vitro microperfusion of cortical TAL, CaSR inhibitors increased calcium reabsorption and paracellular pathway permeability but did not change NaCl reabsorption. We conclude that CaSR is a direct determinant of blood calcium concentration, independent of PTH, and modulates renal tubular calcium transport in the TAL via the permeability of the paracellular pathway. These findings suggest that CaSR inhibitors may provide a new specific treatment for disorders related to impaired PTH secretion, such as primary hypoparathyroidism.
Figures



Comment in
-
Basic research: control of blood calcium concentration by renal CaSR.Nat Rev Nephrol. 2012 Oct;8(10):552. doi: 10.1038/nrneph.2012.173. Epub 2012 Sep 4. Nat Rev Nephrol. 2012. PMID: 22945485 No abstract available.
References
-
- Jaeger P, et al. Animal model of primary hyperparathyroidism. Am J Physiol. 1987;252(6 pt 1):E790–E798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

