Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques
- PMID: 22886365
- PMCID: PMC3495582
- DOI: 10.1007/s00424-012-1135-6
Simultaneous measurement and modulation of multiple physiological parameters in the isolated heart using optical techniques
Abstract
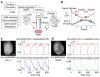
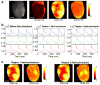
Whole-heart multi-parametric optical mapping has provided valuable insight into the interplay of electrophysiological parameters, and this technology will continue to thrive as dyes are improved and technical solutions for imaging become simpler and cheaper. Here, we show the advantage of using improved 2nd-generation voltage dyes, provide a simple solution to panoramic multi-parametric mapping, and illustrate the application of flash photolysis of caged compounds for studies in the whole heart. For proof of principle, we used the isolated rat whole-heart model. After characterising the blue and green isosbestic points of di-4-ANBDQBS and di-4-ANBDQPQ, respectively, two voltage and calcium mapping systems are described. With two newly custom-made multi-band optical filters, (1) di-4-ANBDQBS and fluo-4 and (2) di-4-ANBDQPQ and rhod-2 mapping are demonstrated. Furthermore, we demonstrate three-parameter mapping using di-4-ANBDQPQ, rhod-2 and NADH. Using off-the-shelf optics and the di-4-ANBDQPQ and rhod-2 combination, we demonstrate panoramic multi-parametric mapping, affording a 360° spatiotemporal record of activity. Finally, local optical perturbation of calcium dynamics in the whole heart is demonstrated using the caged compound, o-nitrophenyl ethylene glycol tetraacetic acid (NP-EGTA), with an ultraviolet light-emitting diode (LED). Calcium maps (heart loaded with di-4-ANBDQPQ and rhod-2) demonstrate successful NP-EGTA loading and local flash photolysis. All imaging systems were built using only a single camera. In conclusion, using novel 2nd-generation voltage dyes, we developed scalable techniques for multi-parametric optical mapping of the whole heart from one point of view and panoramically. In addition to these parameter imaging approaches, we show that it is possible to use caged compounds and ultraviolet LEDs to locally perturb electrophysiological parameters in the whole heart.
Figures



References
-
- Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. - PubMed
-
- Bernardinelli Y, Haeberli C, Chatton JY. Flash photolysis using a light emitting diode: an efficient, compact, and affordable solution. Cell Calcium. 2005;37(6):565–572. - PubMed
-
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

